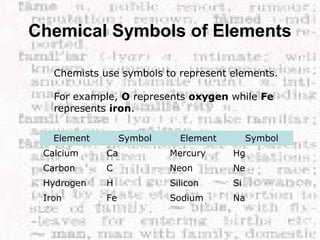
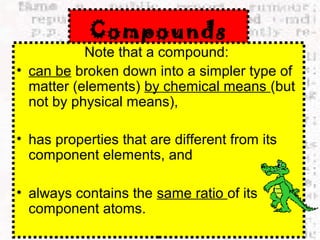
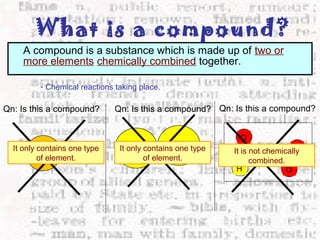
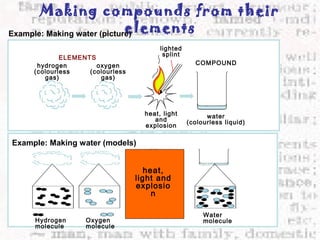
This document discusses the classification of matter into elements, compounds, and mixtures. An element consists of only one type of atom and cannot be separated into simpler substances. A compound contains two or more elements chemically bonded together and has distinct properties from its constituent elements. A mixture is not chemically combined and can be separated into its components by physical means alone. Elements, compounds, and mixtures differ in their composition and the ways they can be separated.