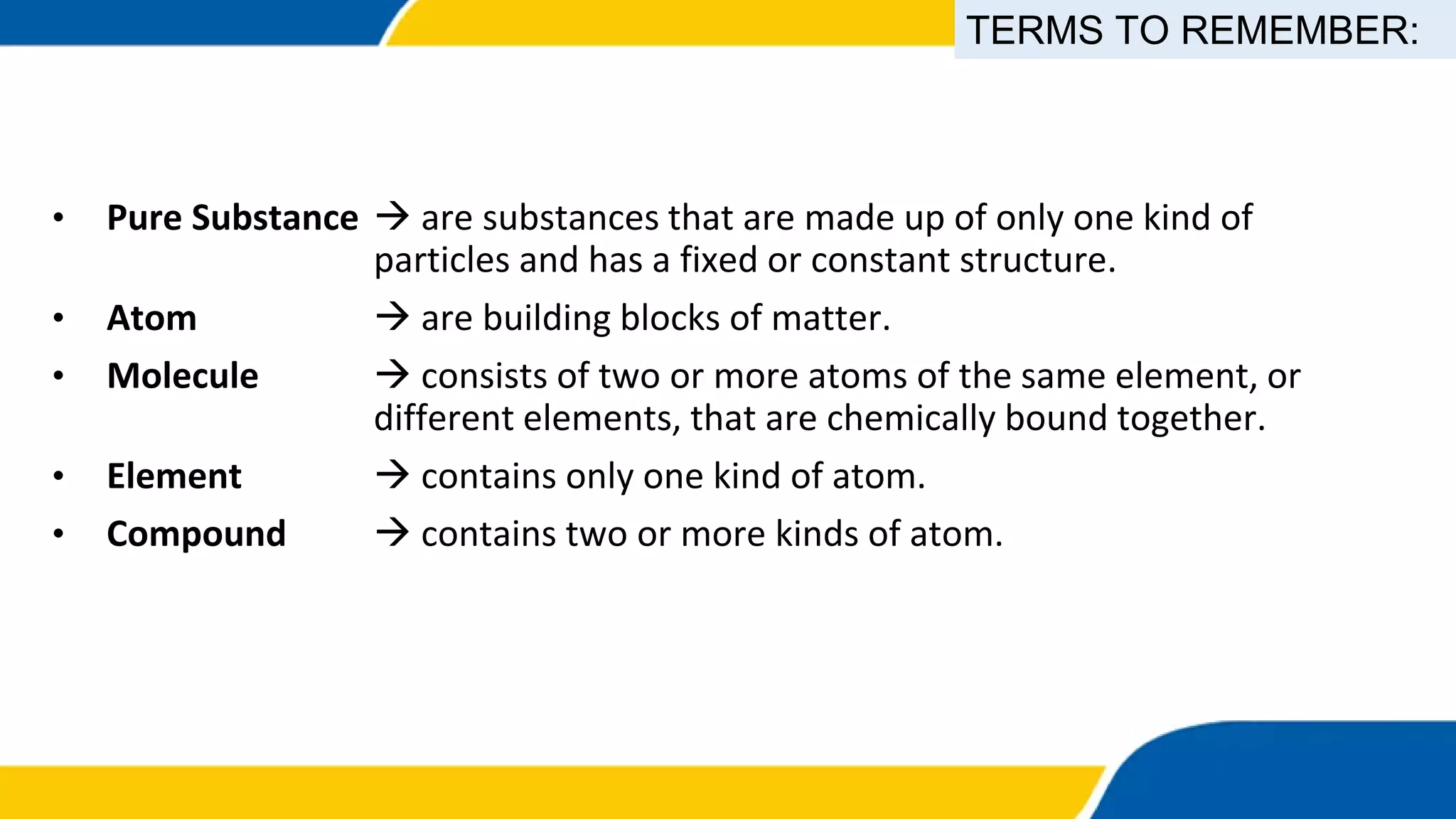
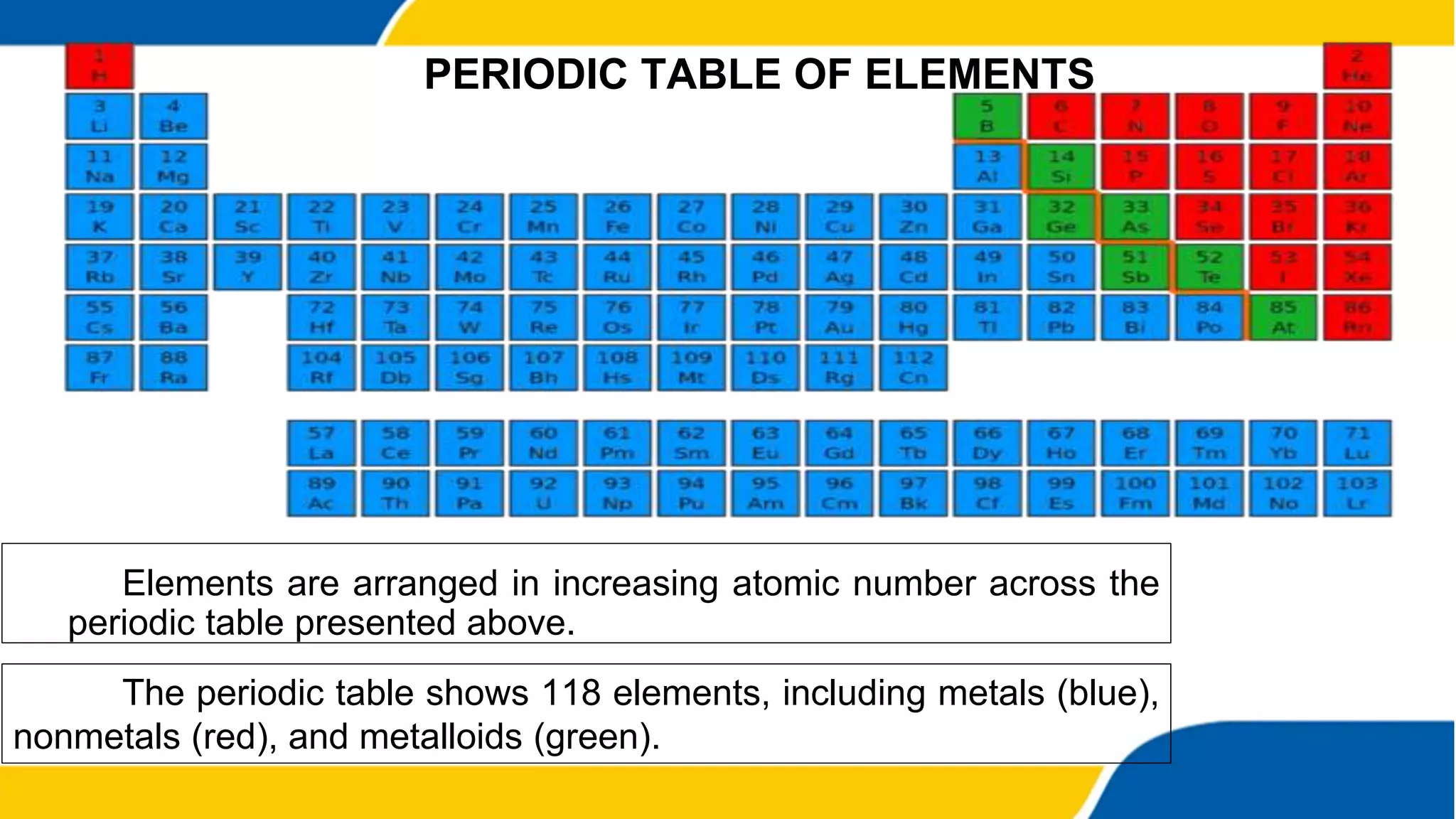
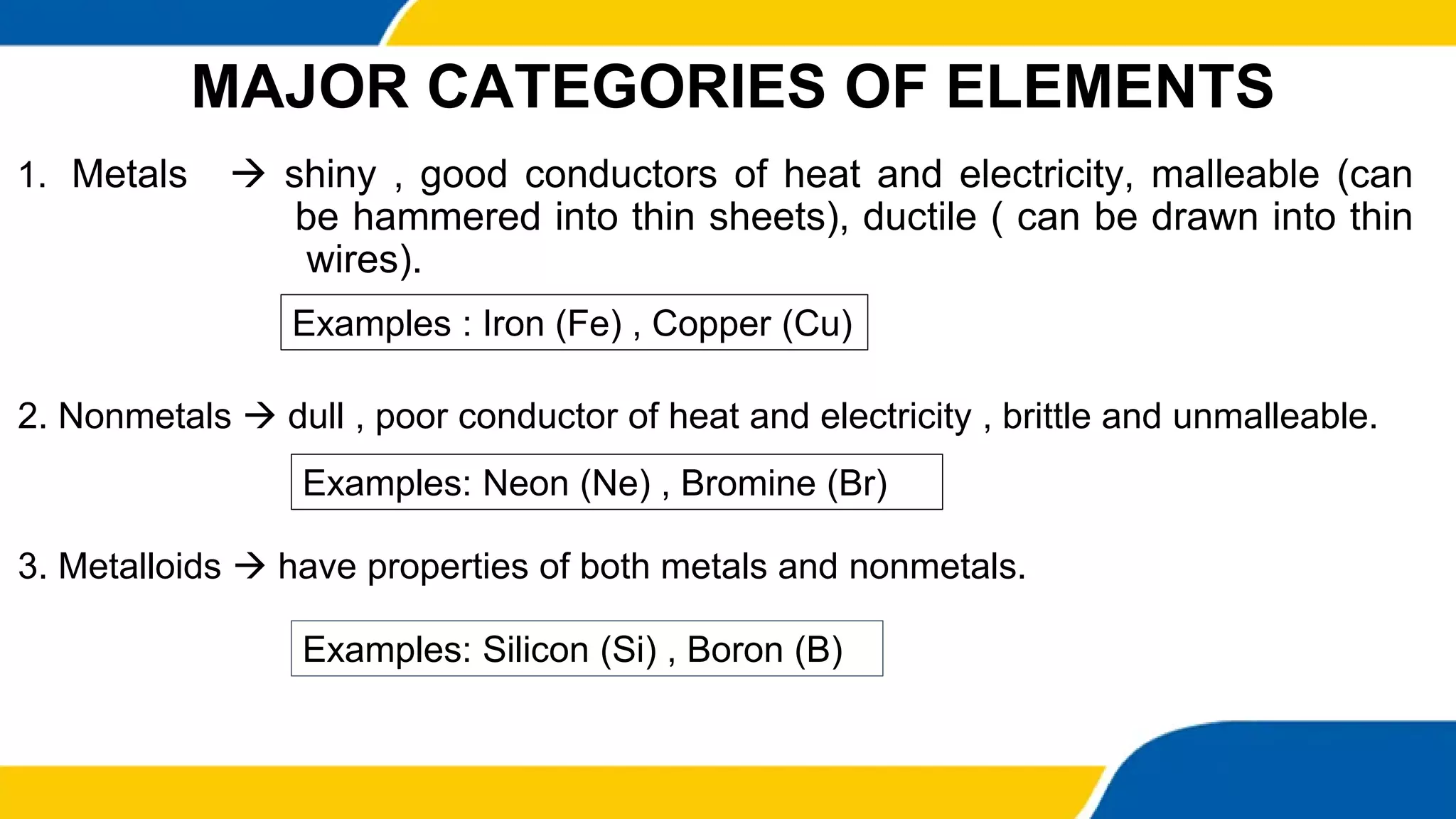
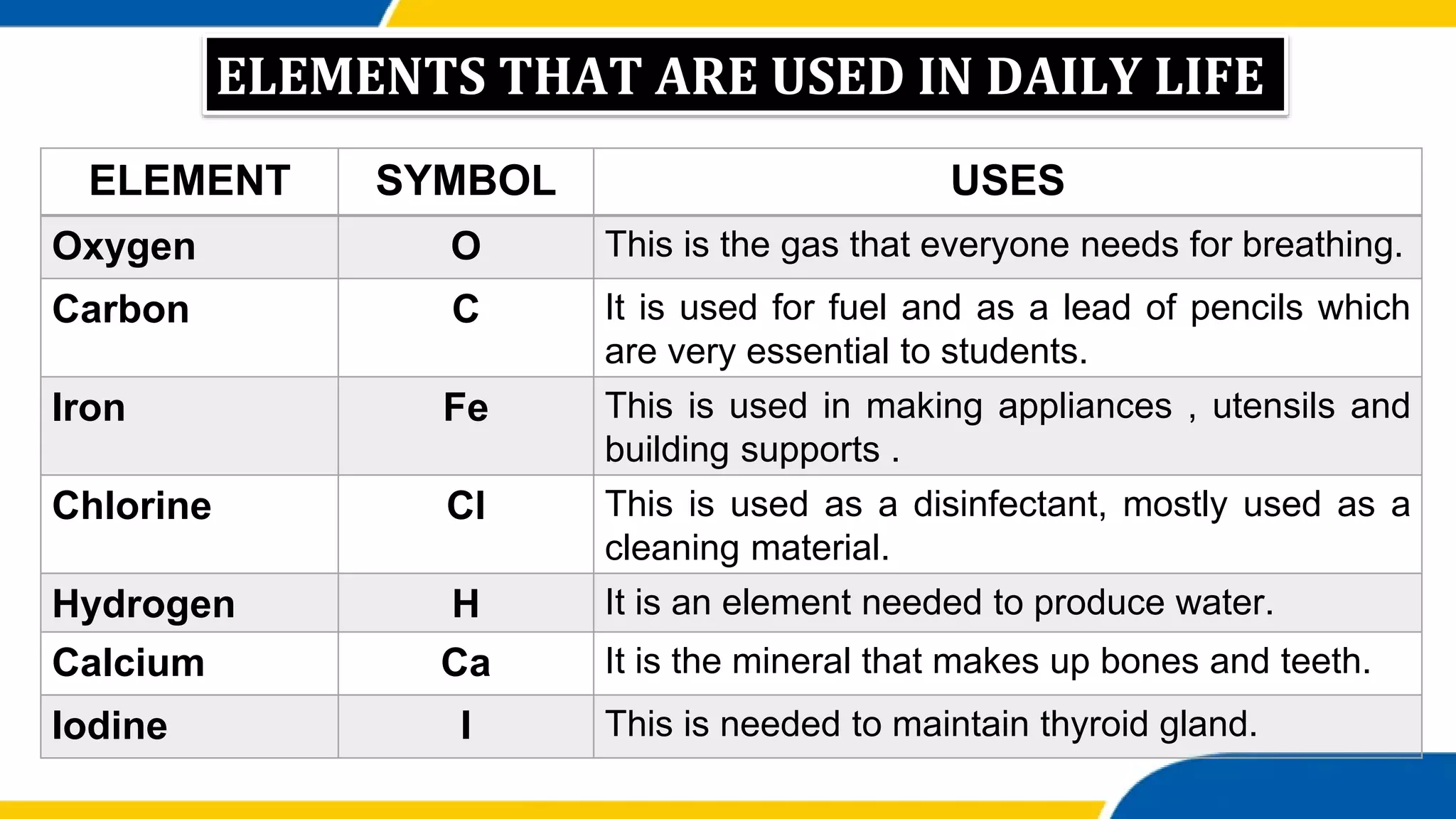
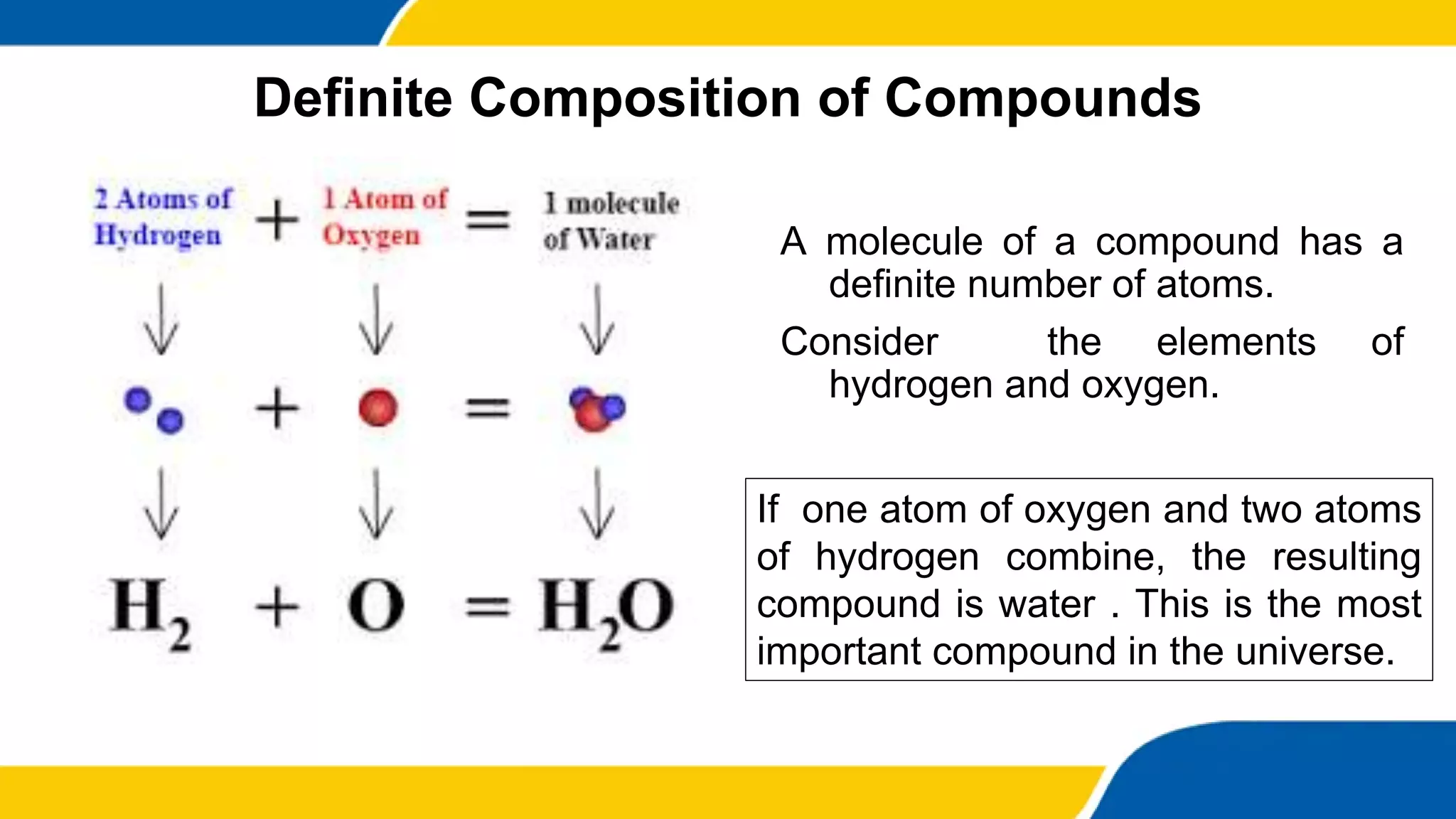
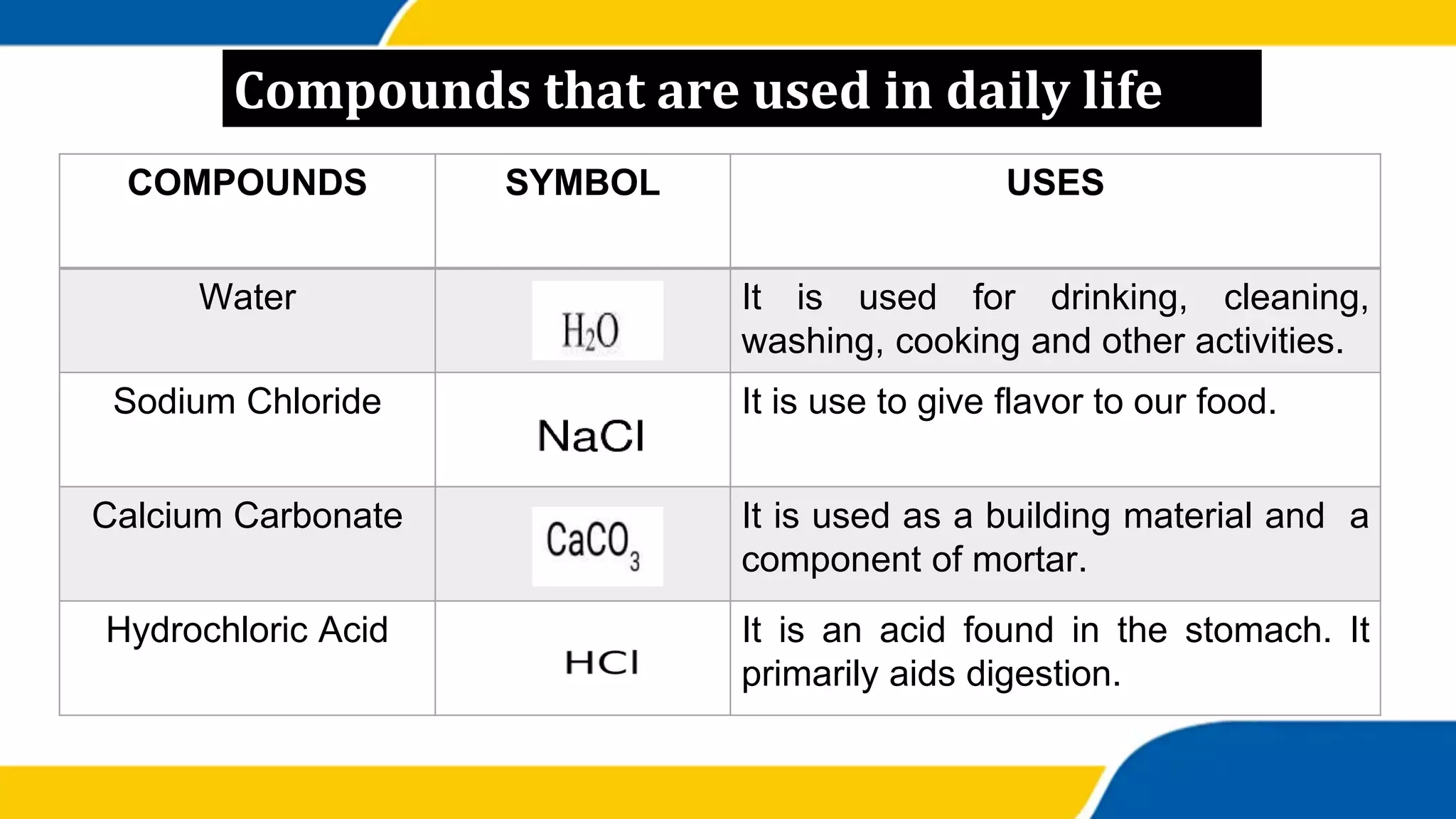
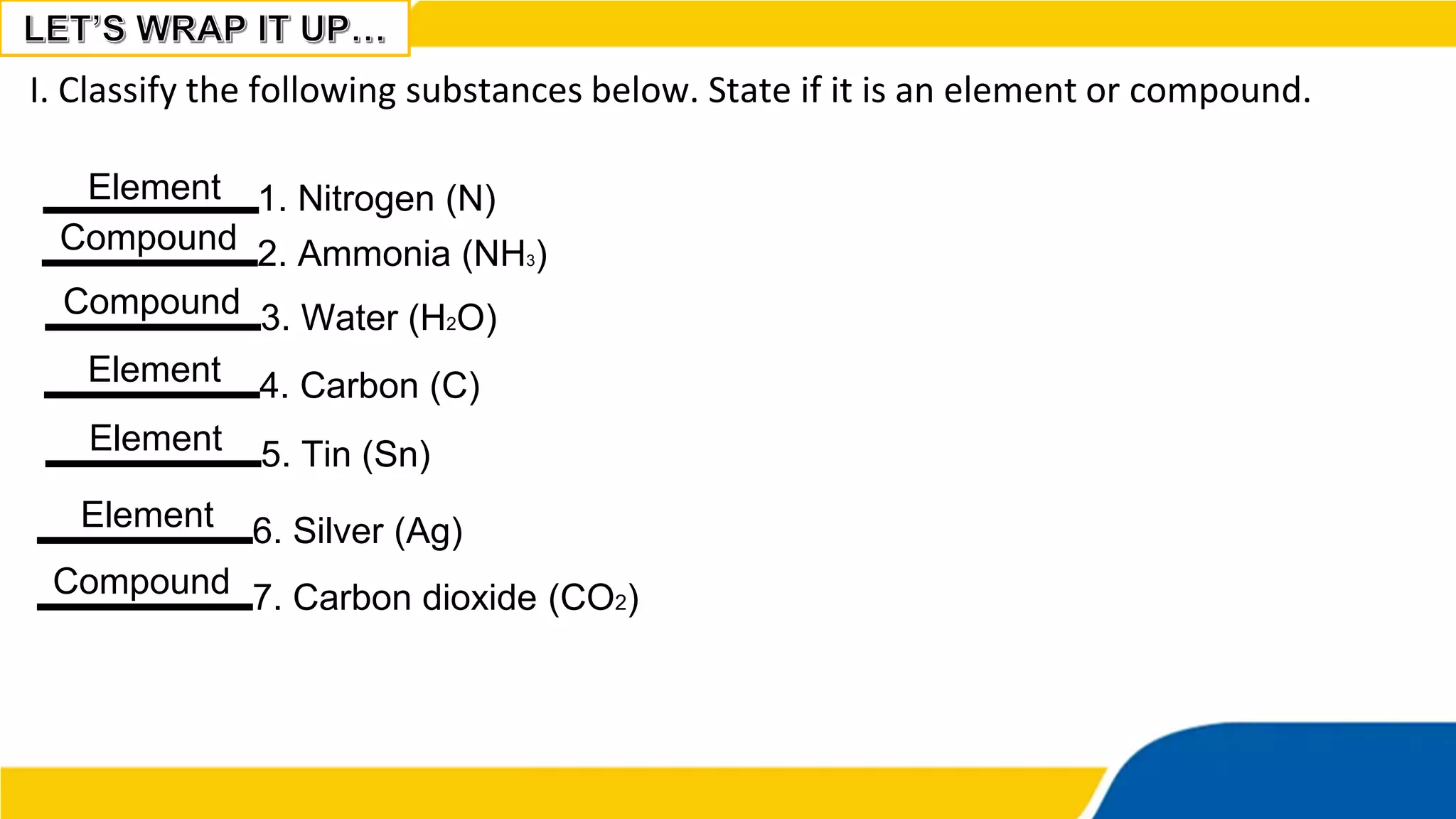
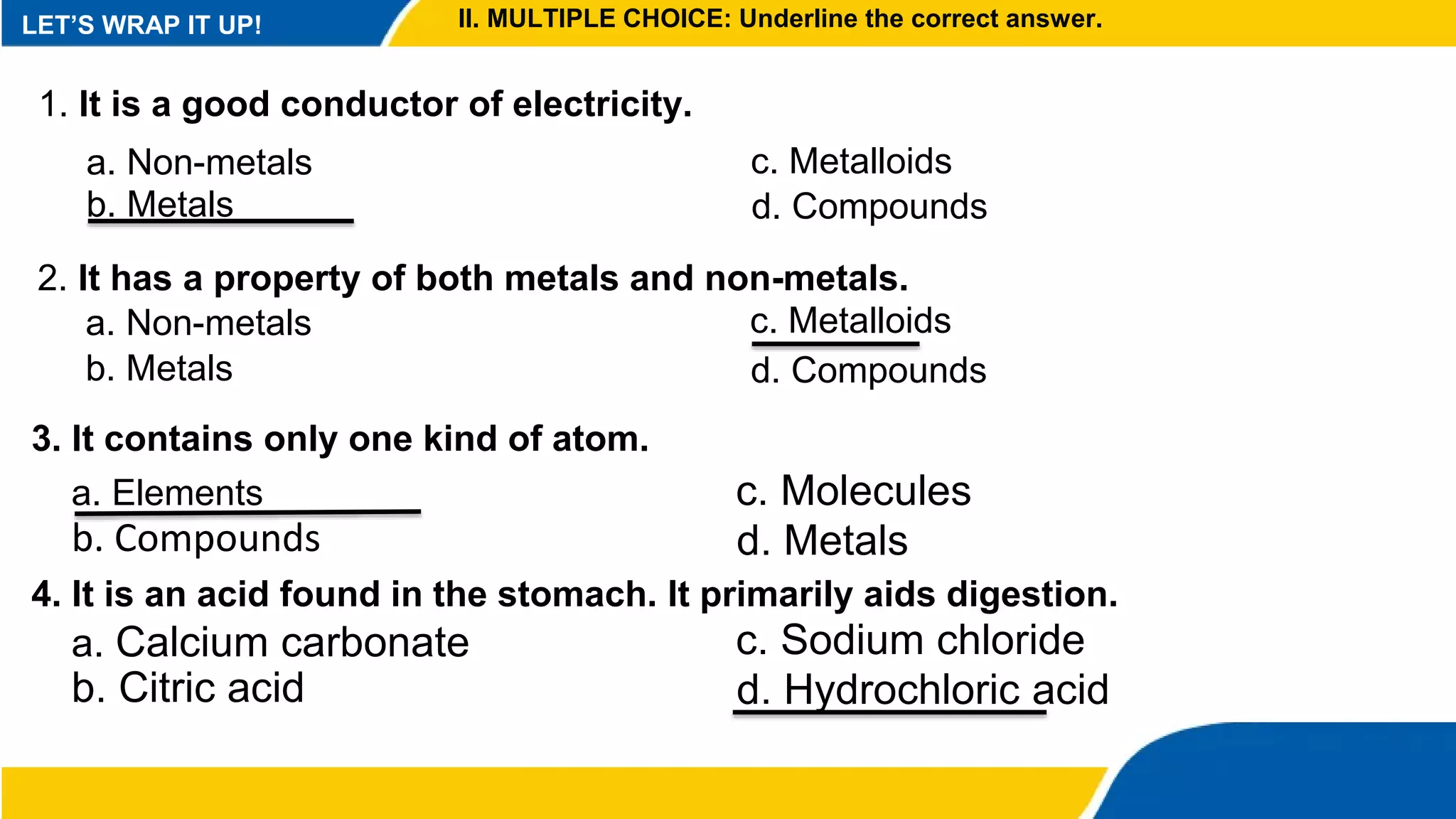
This document provides information about elements and compounds. It begins by defining pure substances as either elements or compounds. Elements contain only one type of atom, while compounds contain two or more different types of atoms bonded together. Examples of common elements and their symbols are provided, along with their locations on the periodic table. Elements are also classified as metals, nonmetals, and metalloids. Examples of important compounds like water and their chemical formulas are also given. Students are then asked to classify substances as elements or compounds and answer multiple choice questions to assess their understanding of the key concepts.