The document summarizes key aspects of the periodic table, including:
1) It describes the historical development of the periodic table by scientists like Lavoisier, Dobereiner, Newlands, Meyer, and Mendeleev.
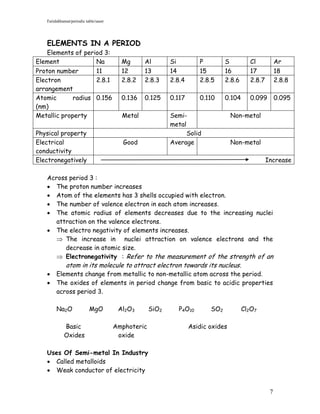
2) It explains the modern arrangement of elements in the periodic table based on proton number and discusses the properties of elements in the same group and period.
3) It provides examples of properties and reactions of representative elements from groups 1, 17, 18 and period 3 of the periodic table. Transition elements and semimetals are also discussed.