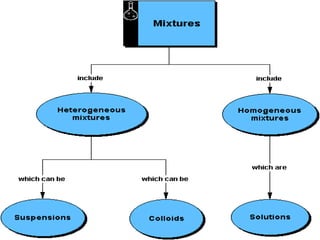
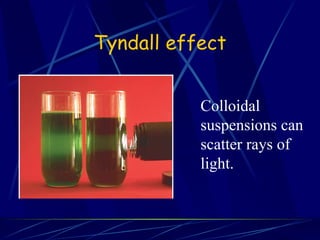
This document compares and contrasts solutions, suspensions, and colloids. It defines each type of mixture and provides examples. Solutions have dissolved particles that do not separate or scatter light, while suspensions are heterogeneous mixtures with insoluble particles that settle out and can be filtered. Colloids have properties of both solutions and suspensions, with small particles that do not dissolve and cannot be separated by filtering. The document gives examples like milk and blood plasma to illustrate colloids.