The document provides information about chemical compounds and formulas:
1. It defines a compound as two or more elements chemically joined together to form a new substance with different properties than the elements.
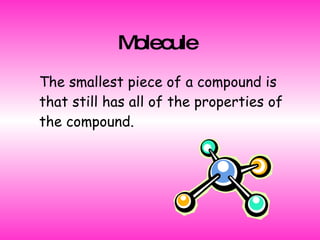
2. It explains that a molecule is the smallest piece of a compound that retains the compound's properties.
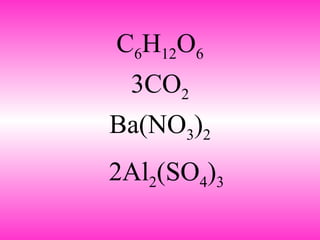
3. It describes how chemical formulas use subscripts to indicate the number of atoms of each element in a compound, and coefficients to indicate the number of molecules.