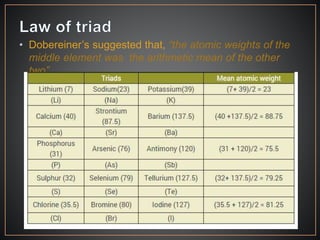
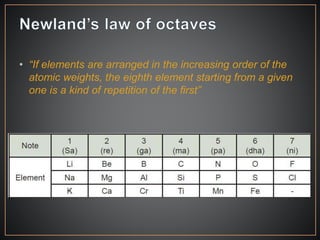
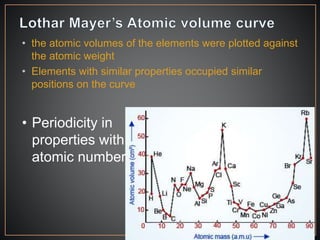
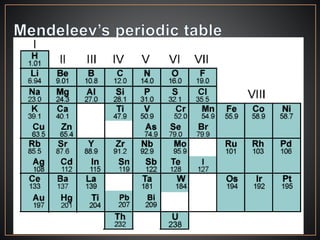
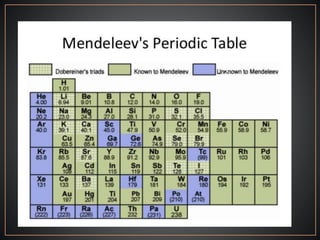
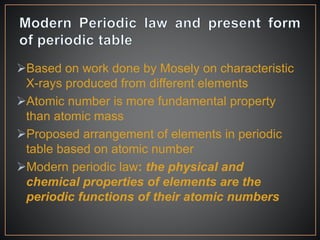
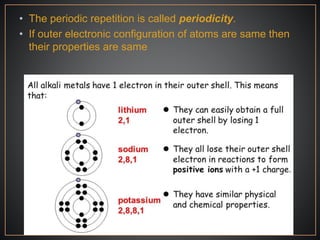
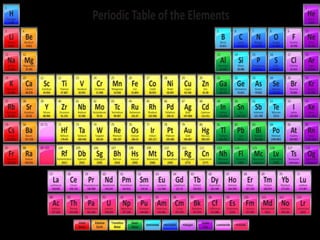
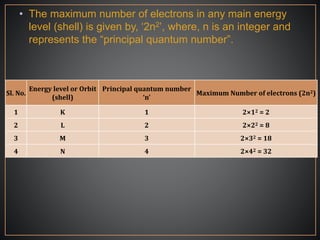
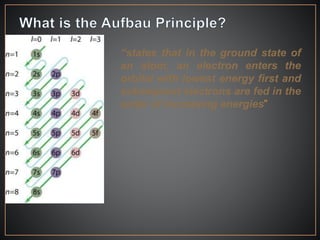
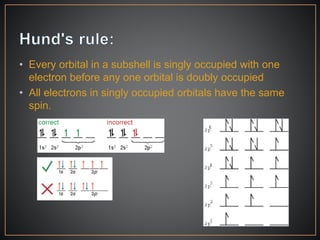
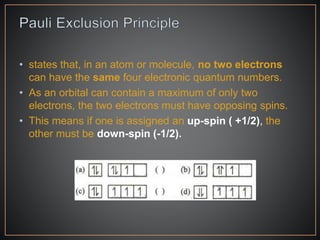
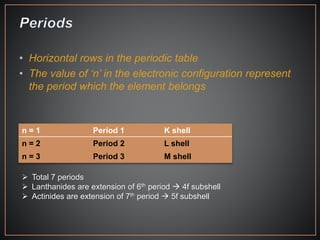
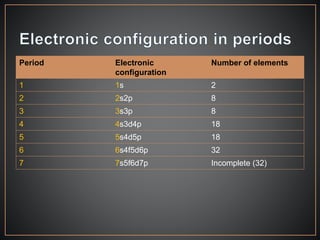
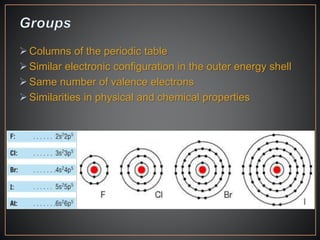
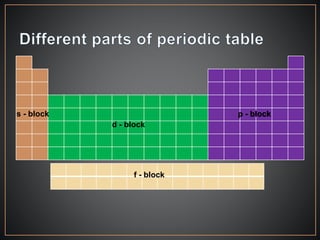
The document summarizes the historical development of the periodic table. It discusses early classification attempts by scientists like Dobereiner, Newlands, and Meyer. Mendeleev organized the 63 known elements into the first recognizable periodic table in 1869 based on periodic trends in their properties. Moseley's work on X-ray spectra led to the modern periodic table being arranged by atomic number instead of atomic mass. The periodic table is now understood to result from the periodic repetition of electronic structures as electrons fill different atomic orbitals in shells.