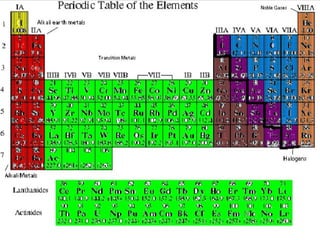
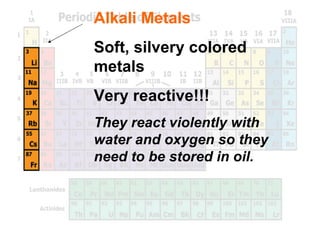
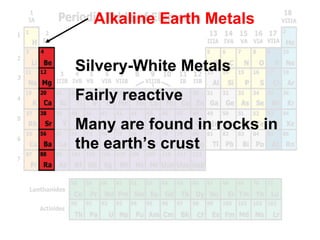
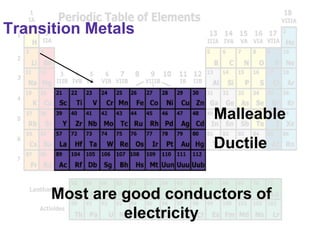
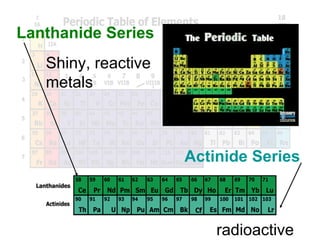
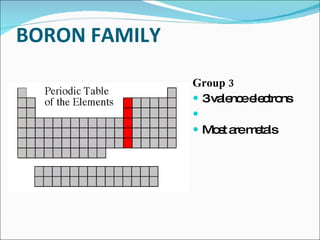
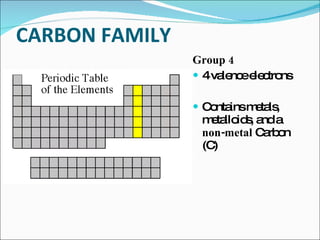
The document outlines the objectives related to the classification of elements in families based on their number of valence electrons and shared characteristics. It details the properties of various families, including alkali metals, alkaline earth metals, transition metals, and others, highlighting their reactivity and physical traits. Additionally, it mentions the boron, carbon, nitrogen, oxygen, and halogen families, indicating the composition and reactivity of elements within these groups.