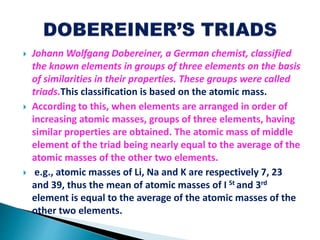
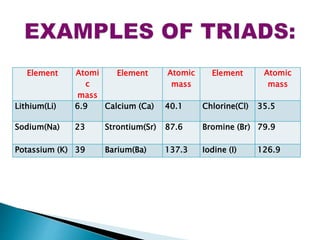
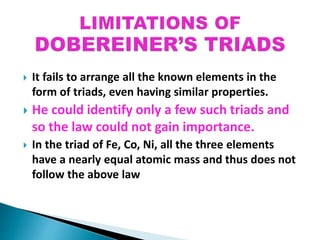
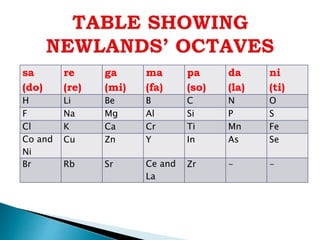
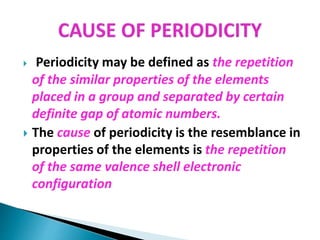
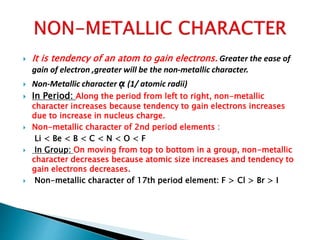
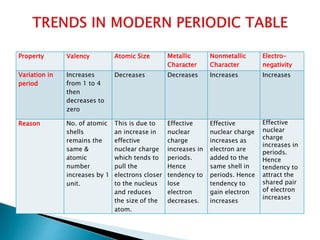
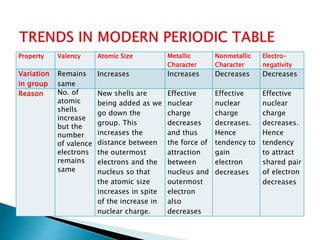
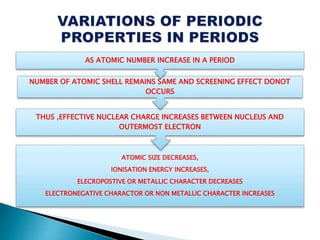
The document details the development of the periodic table, starting from early classification attempts by Dobereiner and Newlands, to Mendeleev's arrangement based on atomic mass and properties. Mendeleev's work highlighted periodicity and allowed predictions of undiscovered elements, though it had limitations like the placement of hydrogen and isotopes. Modern periodicity is based on atomic numbers as proposed by Moseley, which corrected previous shortcomings and established a systematic framework for understanding elemental properties.