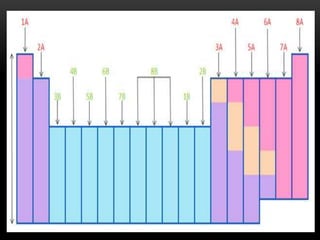
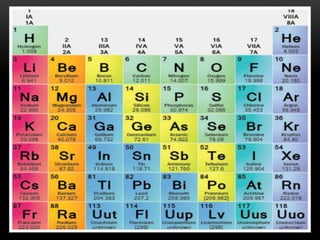
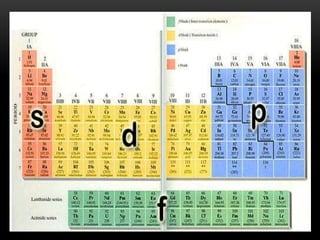
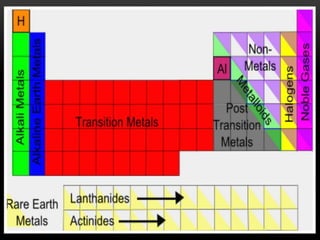
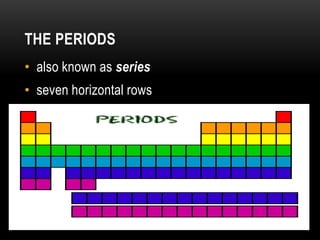
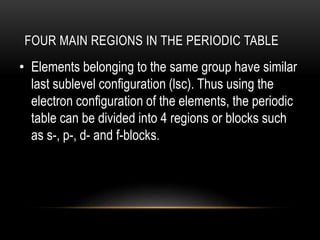
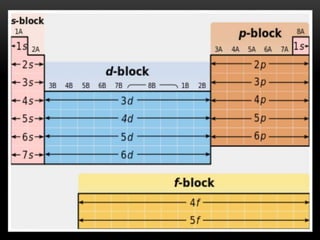
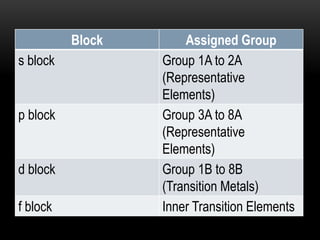
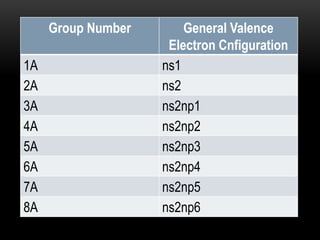
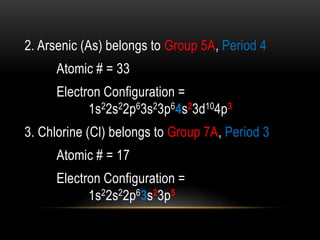
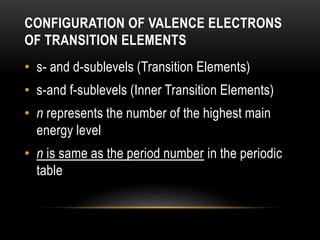
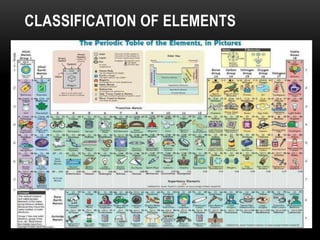
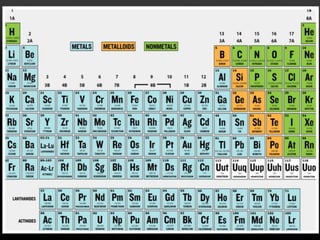
The document discusses the structure and organization of the modern periodic table. Elements are arranged horizontally in order of increasing atomic number and grouped vertically into columns based on their electron configurations. The periodic table is divided into blocks including s-, p-, d- and f-blocks. An element's position in the table is determined by its atomic number and electron configuration, especially the outermost electrons.