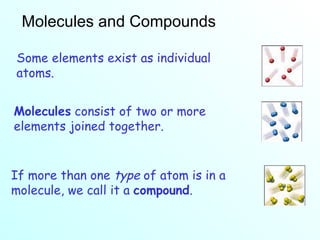
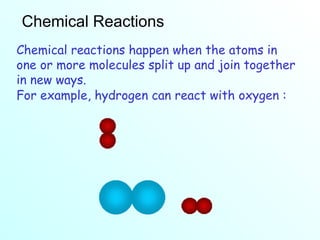
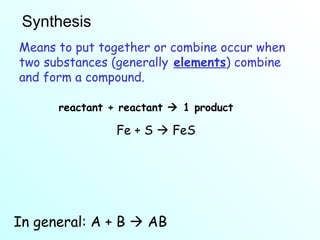
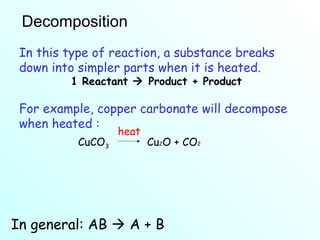
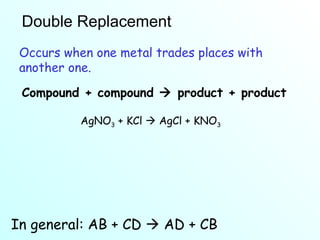
The document discusses chemical reactions and the four main types: synthesis, decomposition, single replacement, and double replacement. It defines key terms like reactants and products. Synthesis reactions combine two elements to form a compound. Decomposition reactions involve a single reactant breaking down into simpler products. Single replacement reactions involve one element replacing another in a compound. Double replacement reactions swap ions between two compounds. The law of conservation of mass is also mentioned.