The document discusses the history and development of the periodic table. It explains that:
1) Elements have been organized into the periodic table based on their atomic weights and properties in order to systematically study and predict chemical behavior.
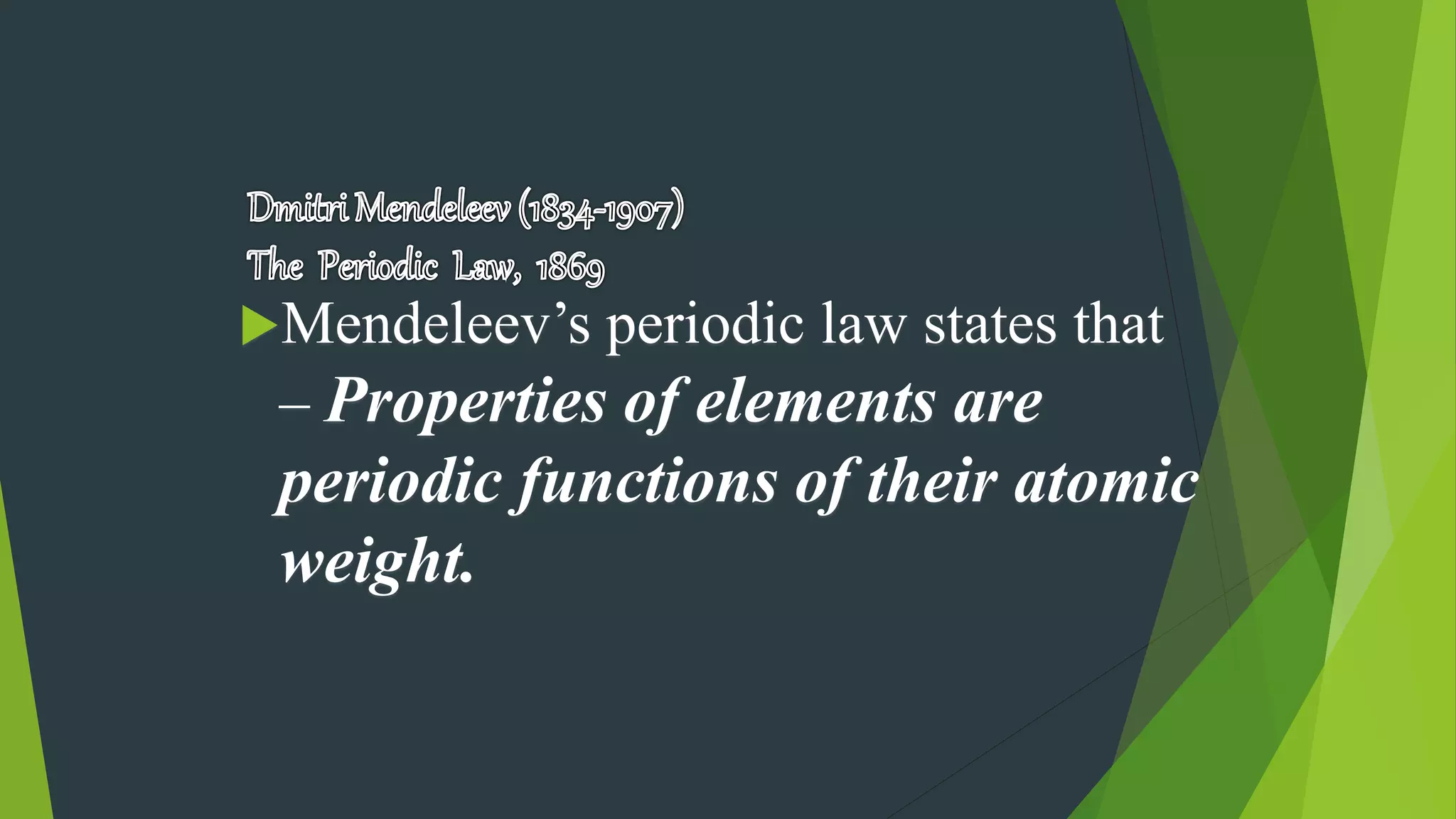
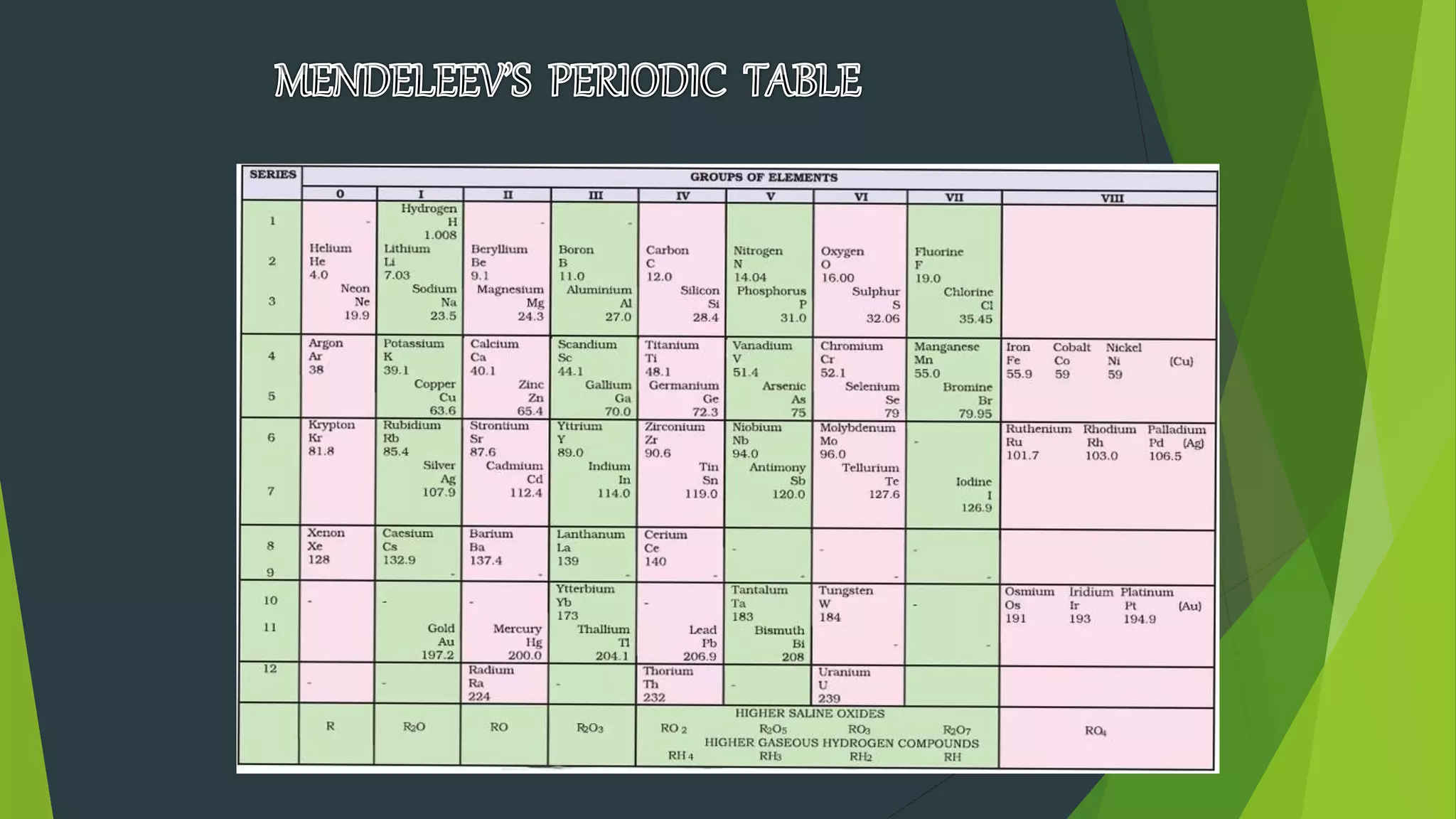
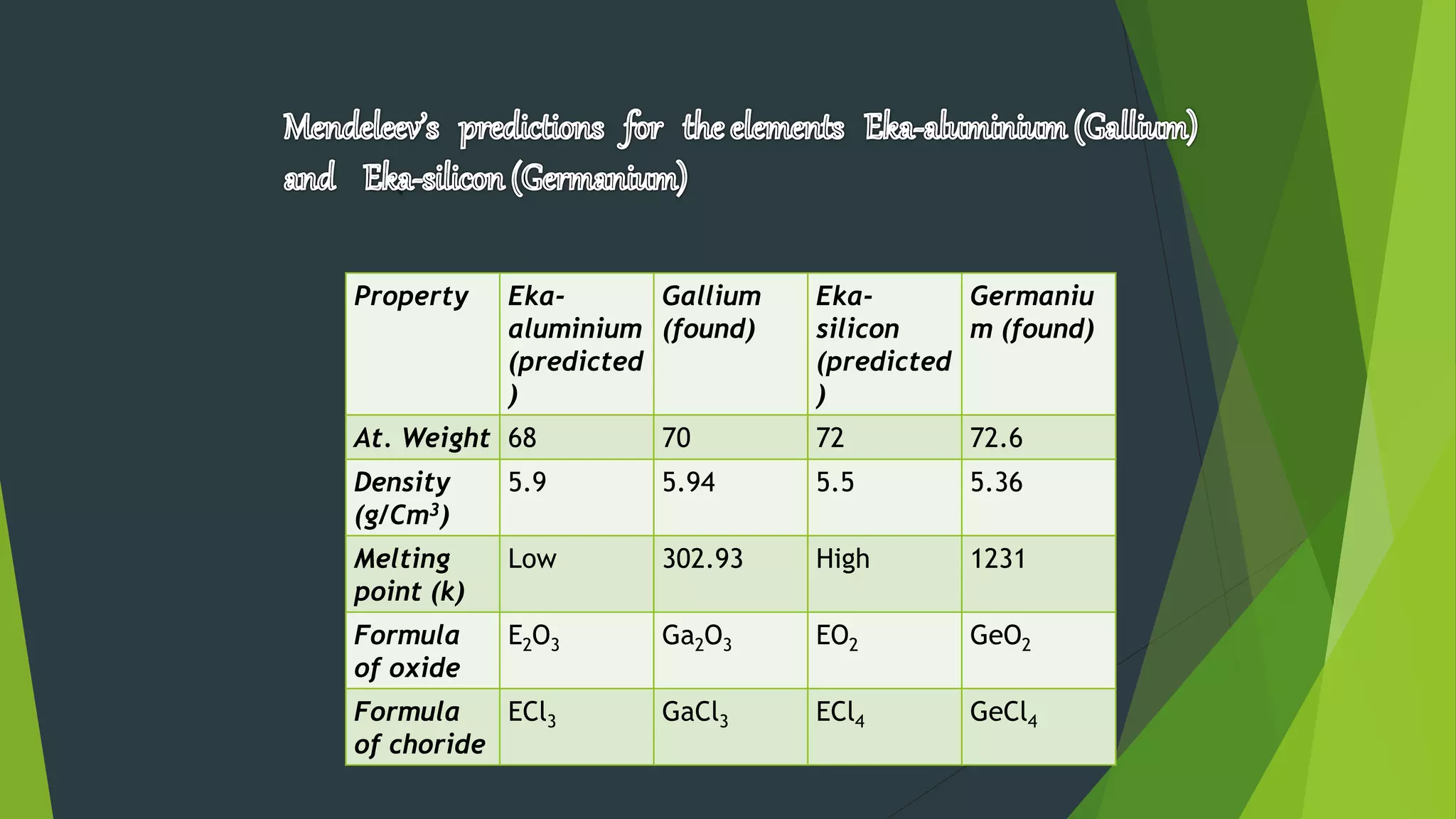
2) Early periodic tables were proposed by scientists like Dobereiner, Newlands, and Mendeleev based on trends in atomic weights, though Mendeleev's 1869 table was the most successful in predicting new elements.
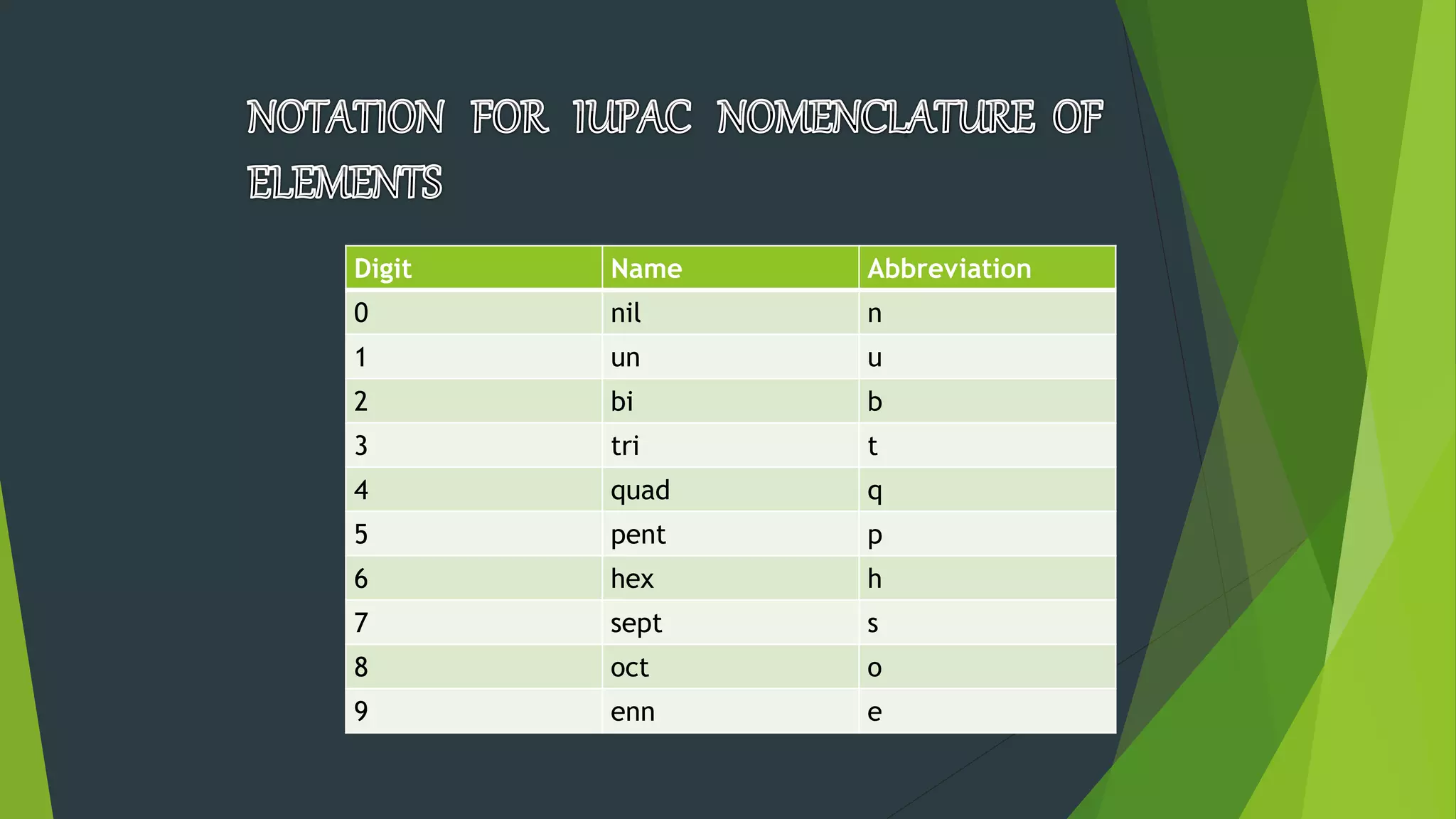
3) Modern periodic tables are arranged by atomic number according to the periodic law proposed by Moseley, with elements grouped into blocks based on their electron configurations. The table better explains trends in physical and chemical properties.

























![ATOMIC
No.
SYMBOL ELECTRONIC COIGUNFRATION
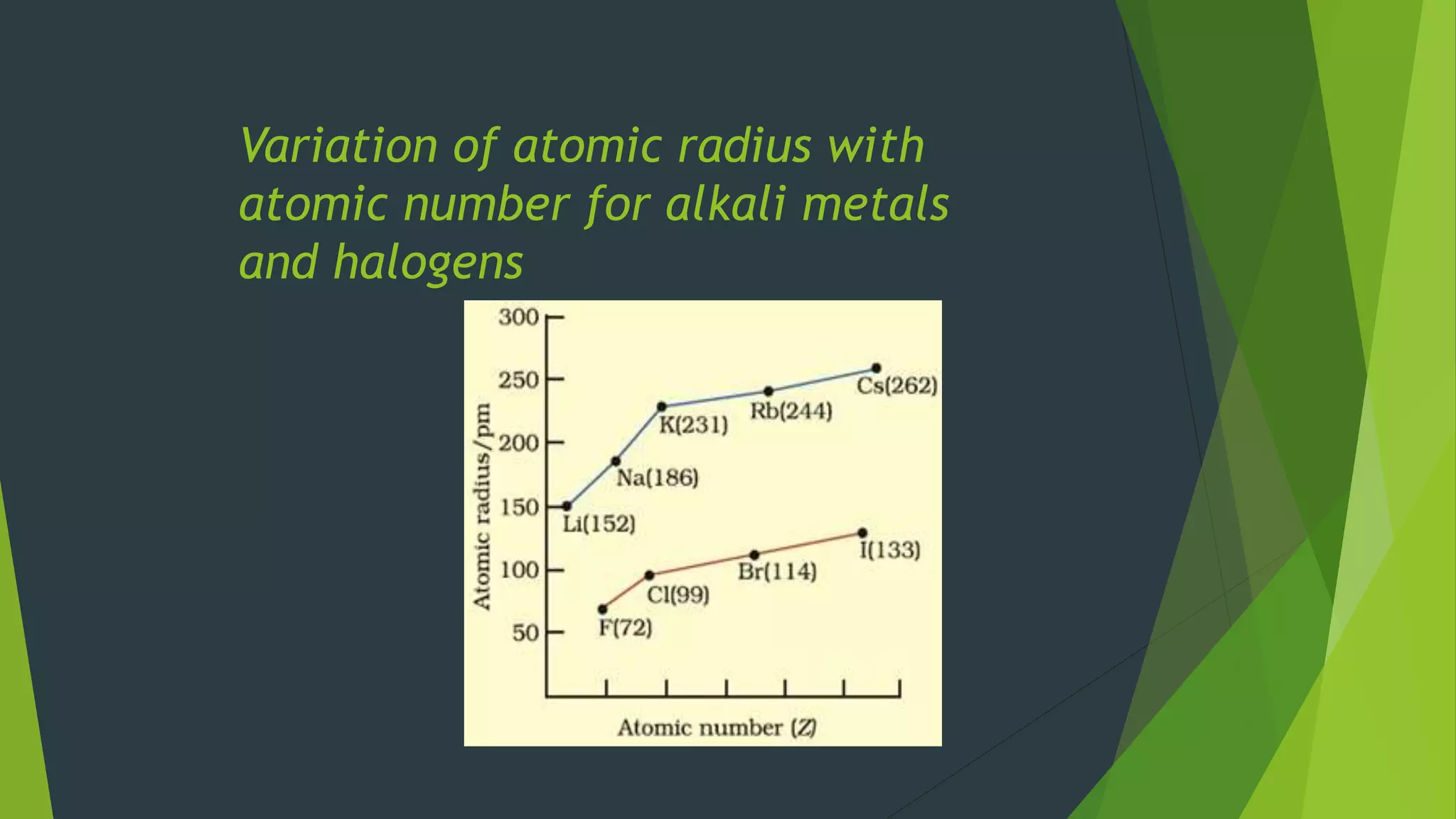
3 Li 1s22s1(or)[He]2s1
11 Na 1s22s22p63s1(or)[Ne]3s1
19 K 1s22s22p63s23p64s1(or)[Ar]4s1
37 Rb 1s22s22p6sup3s23p63d104s24p65s1(or)[Kr]5s1
55 Cs 1s22s22p63s23p63d104s24p64d105s25p66s1(or)[Xe]6s1
87 Fr [Rn]7s1](https://image.slidesharecdn.com/chemistry-151220071034/75/Chemistry-class11-CLASSIFICATION-OF-ELEMENTS-AND-PERIODICITY-IN-PROPERTIES-30-2048.jpg)








![ The elements Z = 117 and 120 have not yet been discovered. In which family / group
would you place these elements and also give the electronic configuration in each
case.
In the periodic table the element with Z = 117, would belong to the halogen family
(Group 17) and the electronic configuration would be [Rn] 5f146d107s27p5. The element
with Z = 120, will be placed in Group 2 (alkaline earth metals), and will have the
electronic configuration [Uuo]8s2.](https://image.slidesharecdn.com/chemistry-151220071034/75/Chemistry-class11-CLASSIFICATION-OF-ELEMENTS-AND-PERIODICITY-IN-PROPERTIES-41-2048.jpg)





















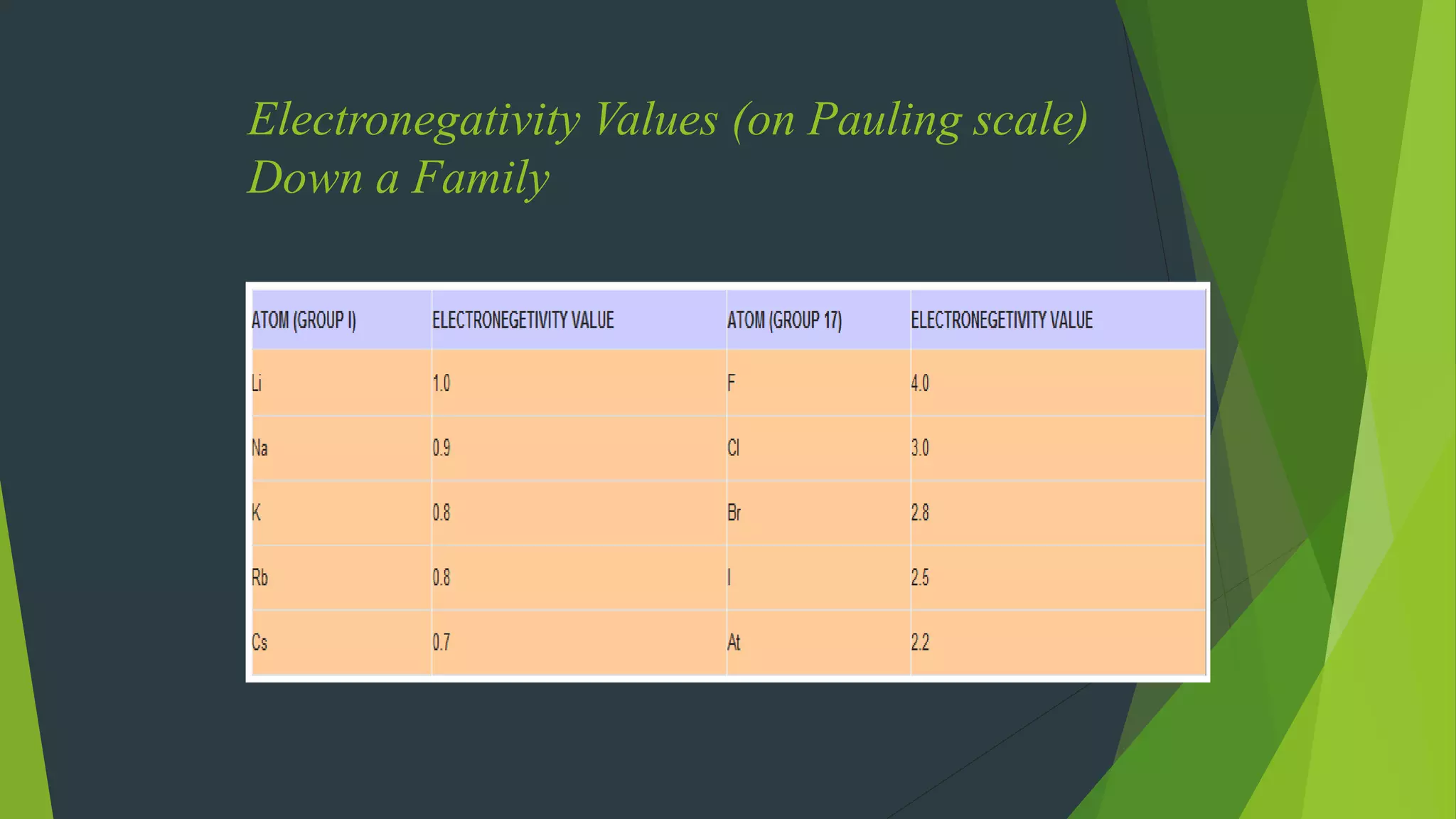









![ Are the oxidation state and covalency of Al in [AlCl(H2O)5]2+ same ?
No. The oxidation state of Al is +3 and the covalency is 6.](https://image.slidesharecdn.com/chemistry-151220071034/75/Chemistry-class11-CLASSIFICATION-OF-ELEMENTS-AND-PERIODICITY-IN-PROPERTIES-79-2048.jpg)





