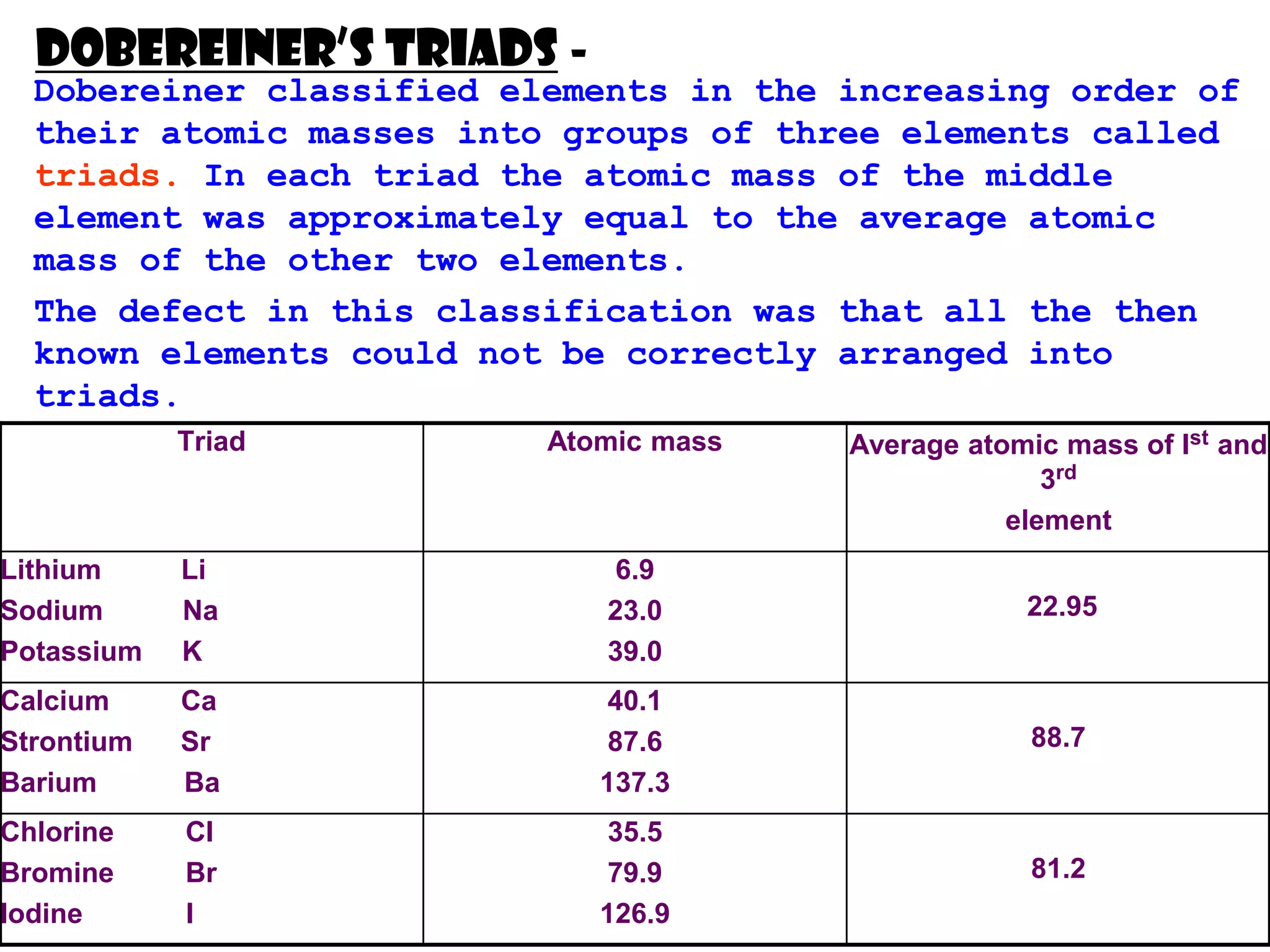
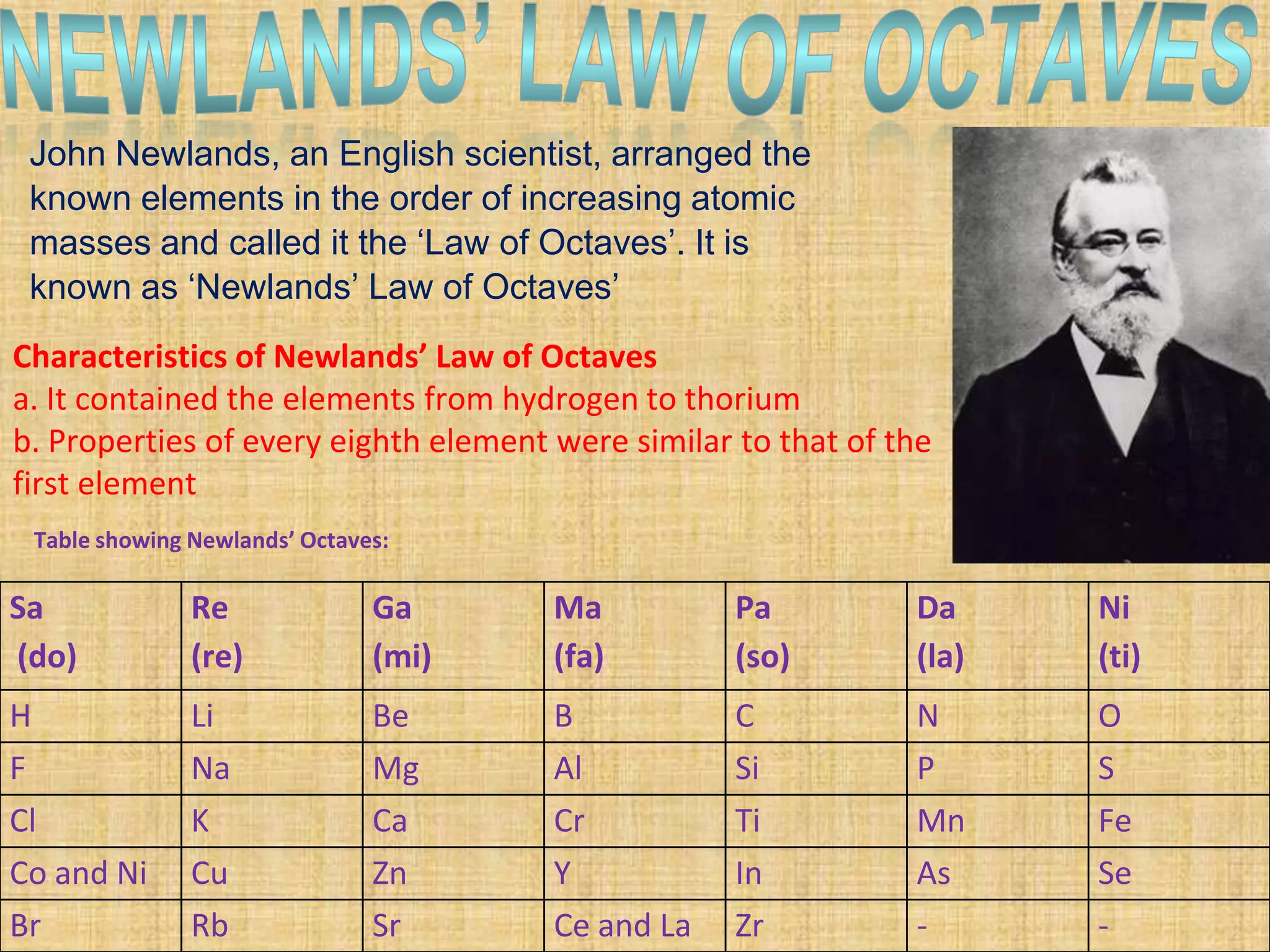
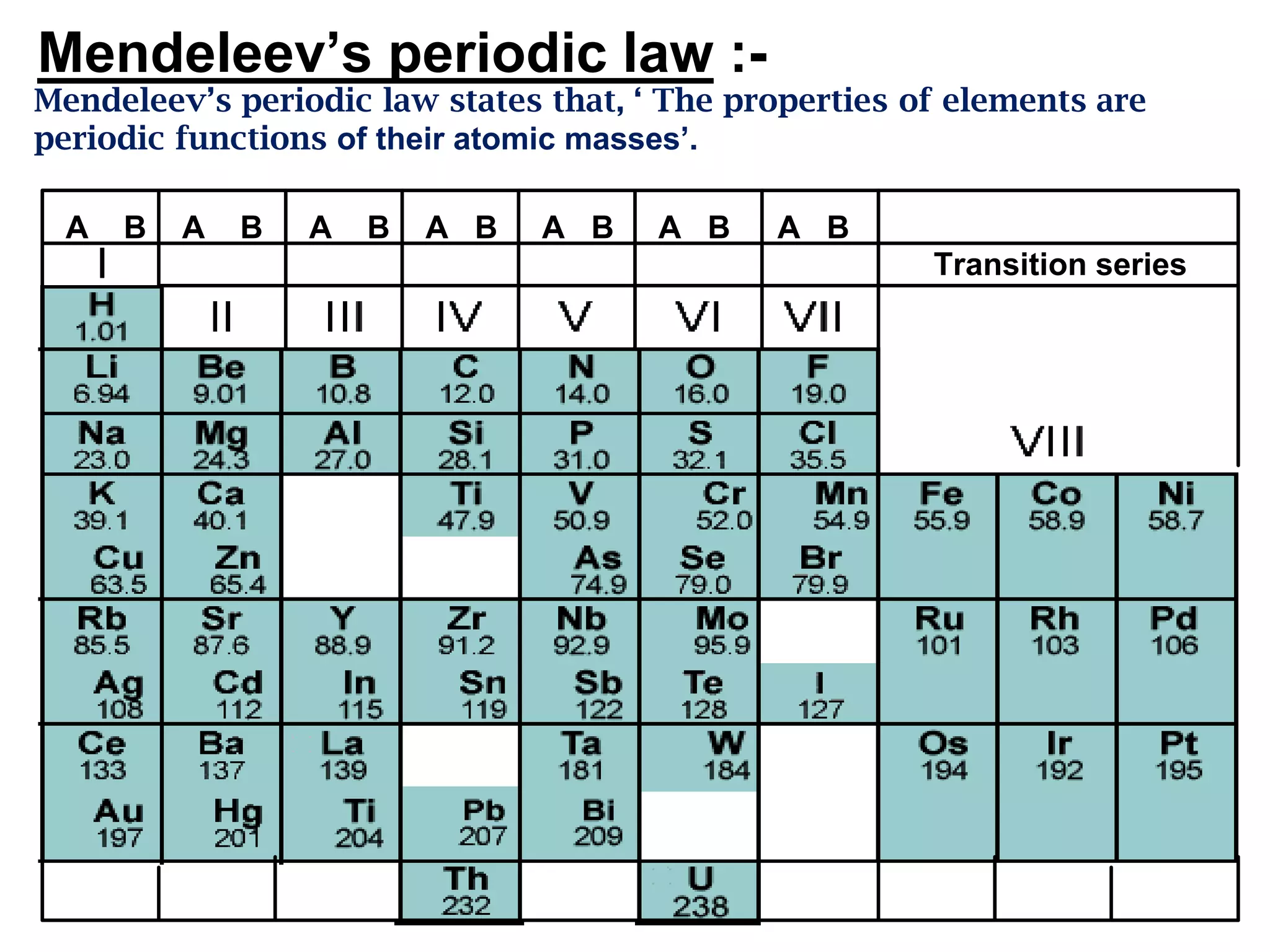
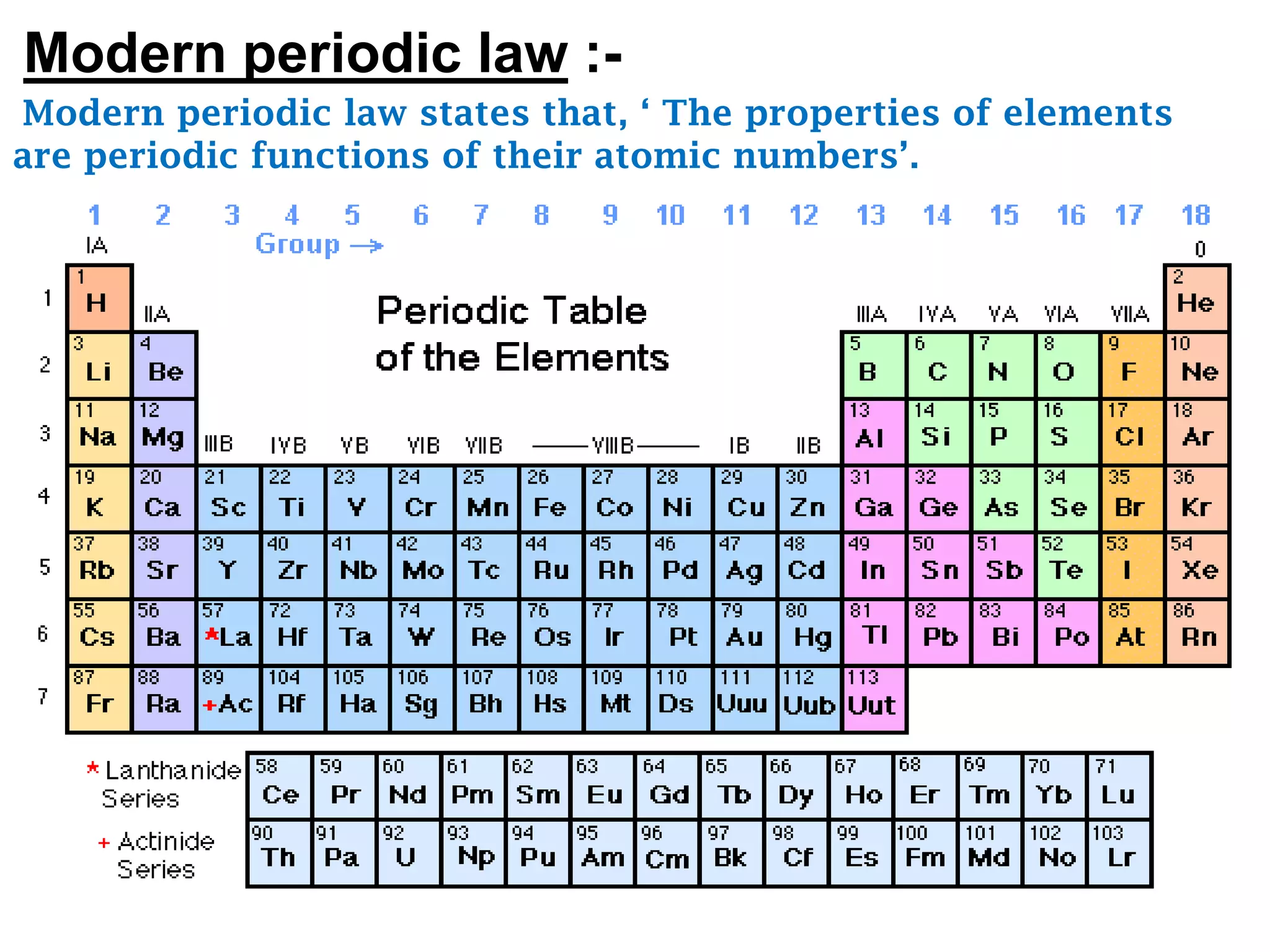
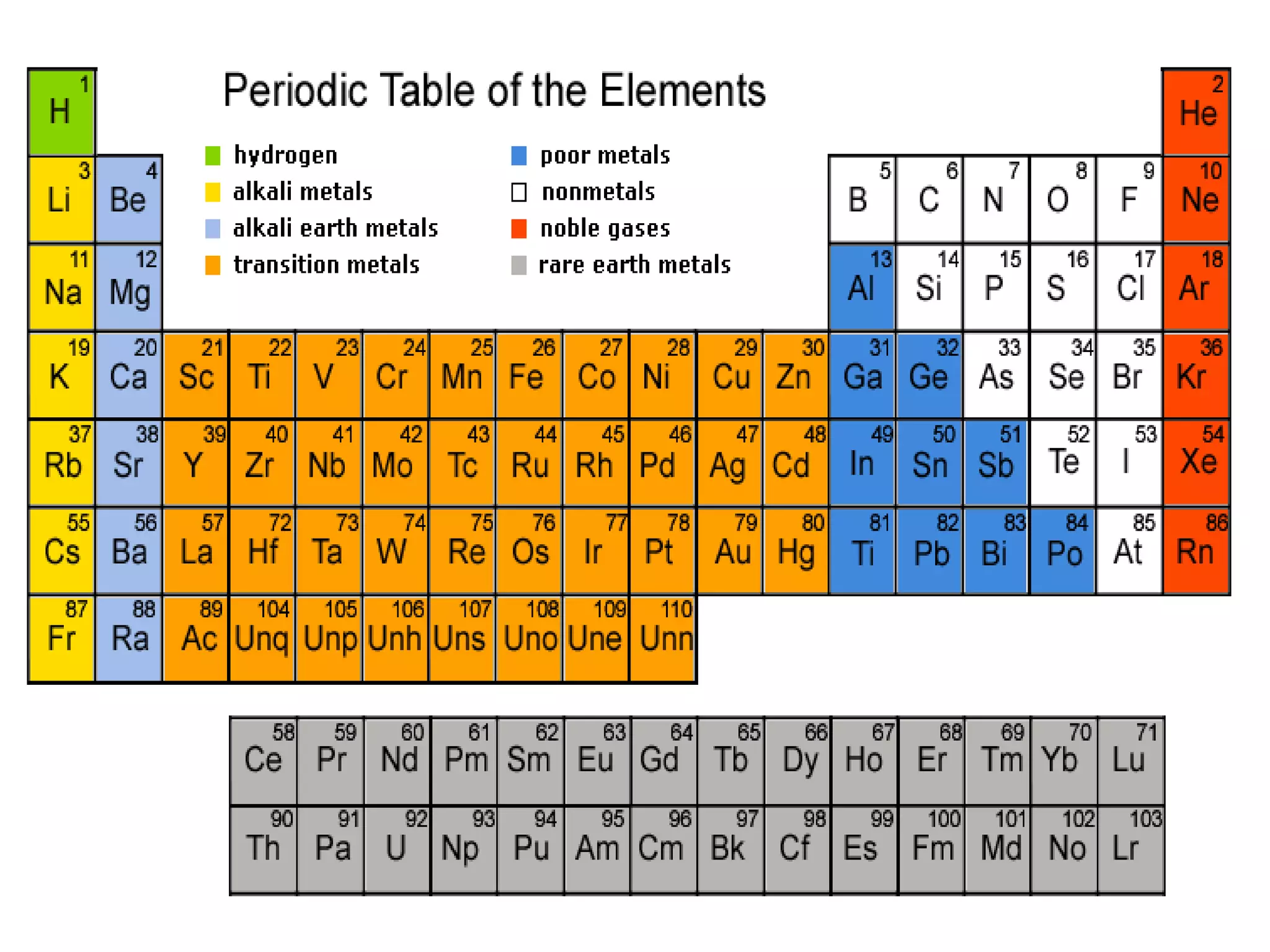
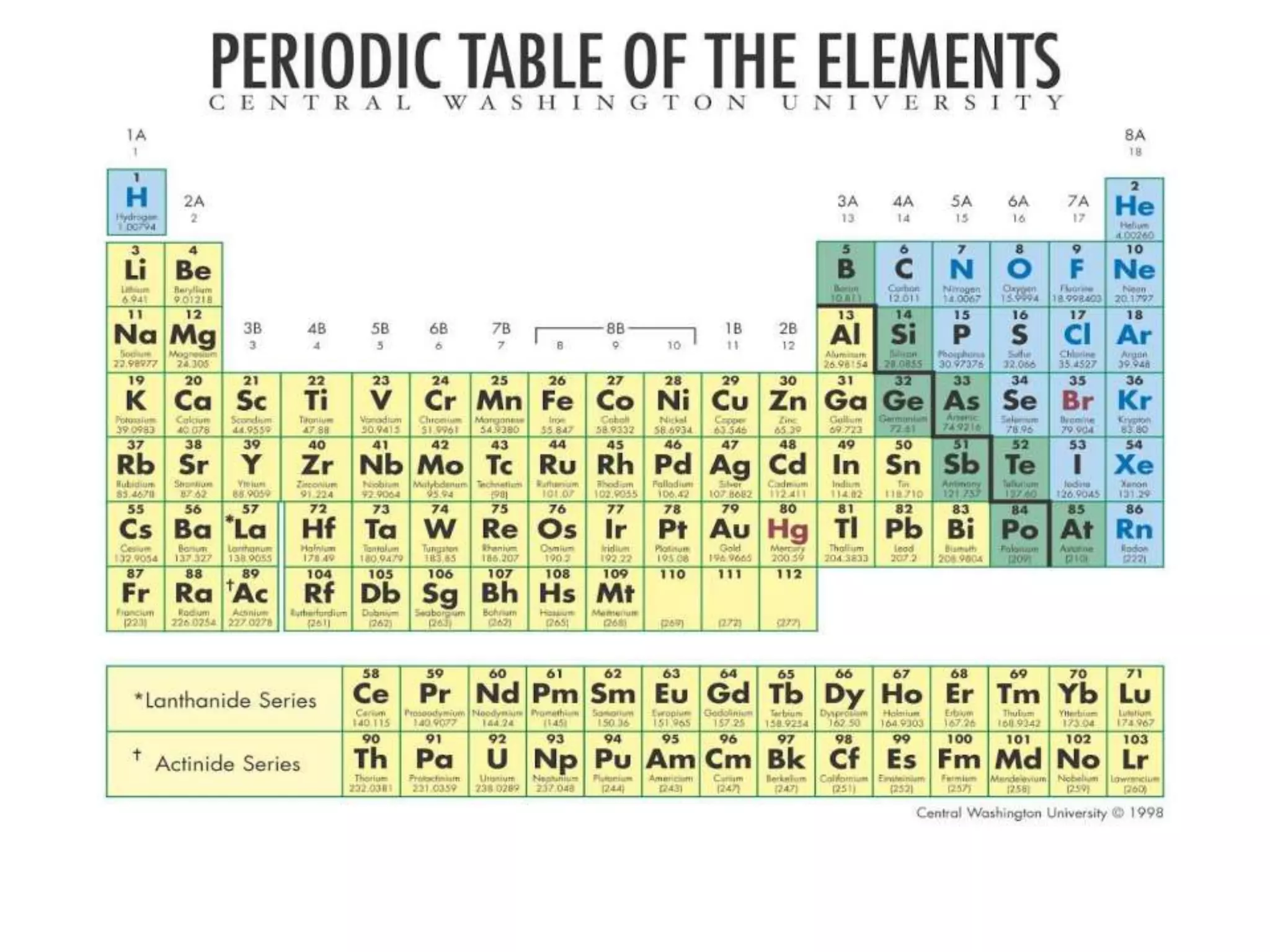
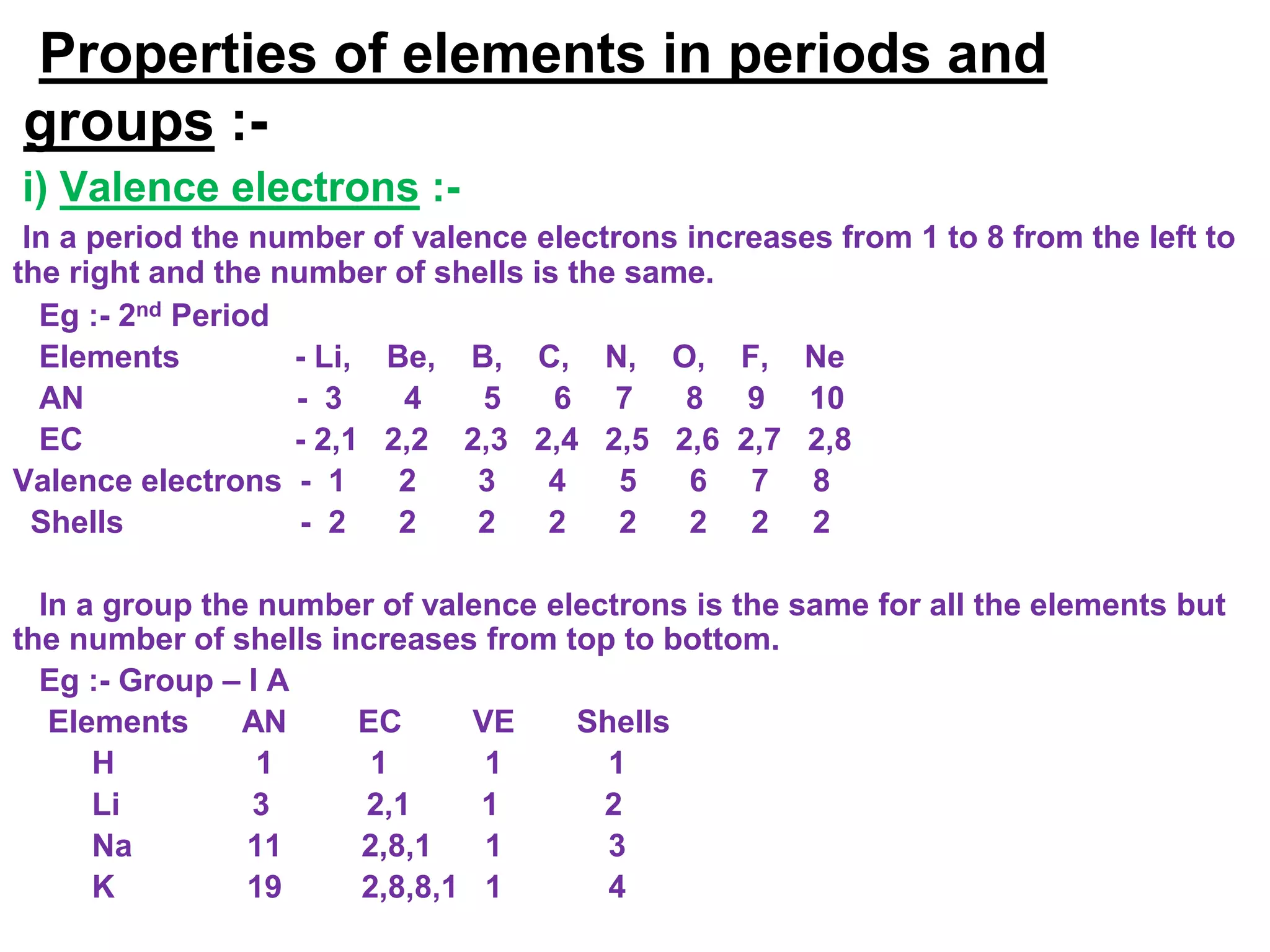
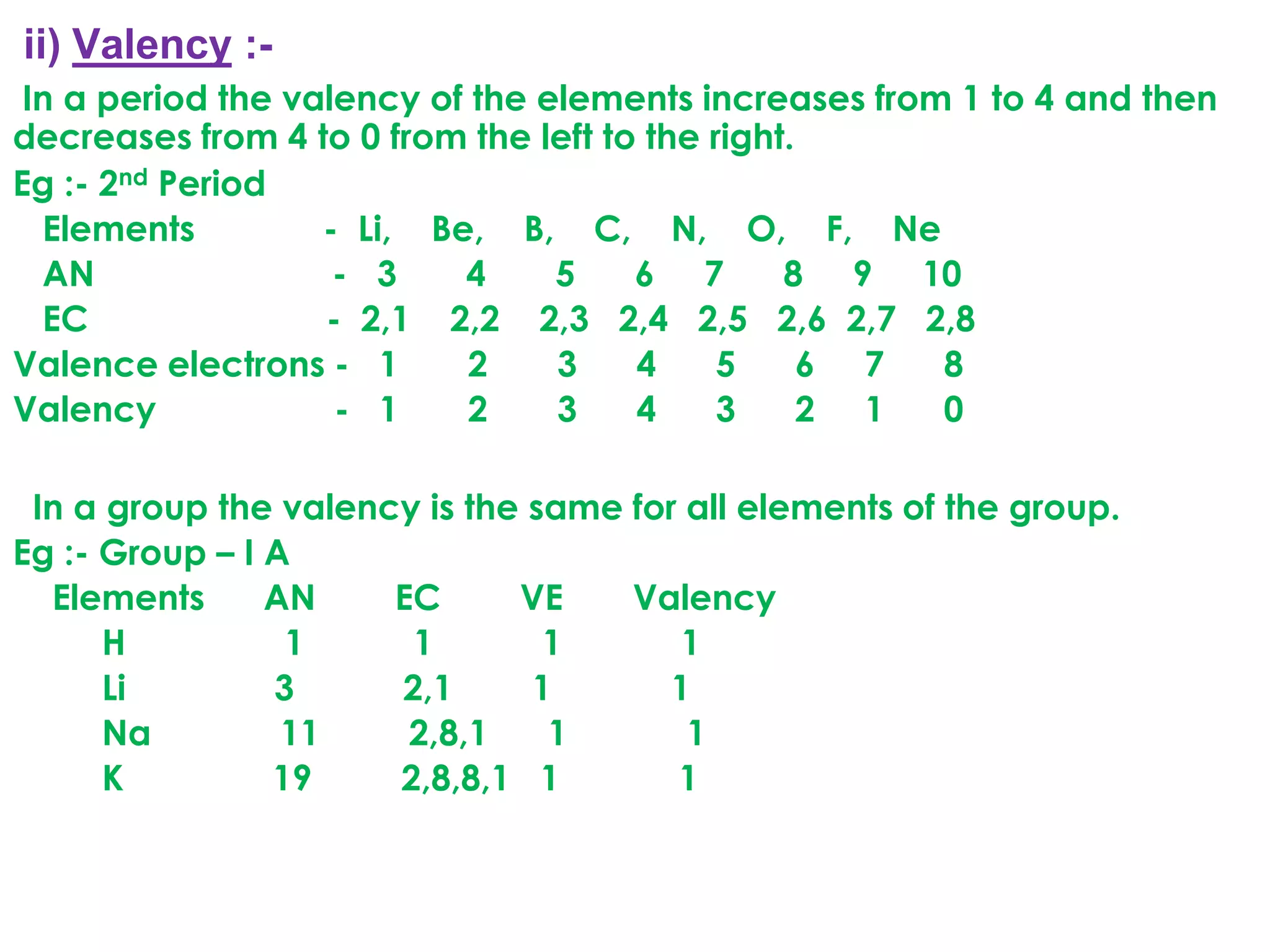
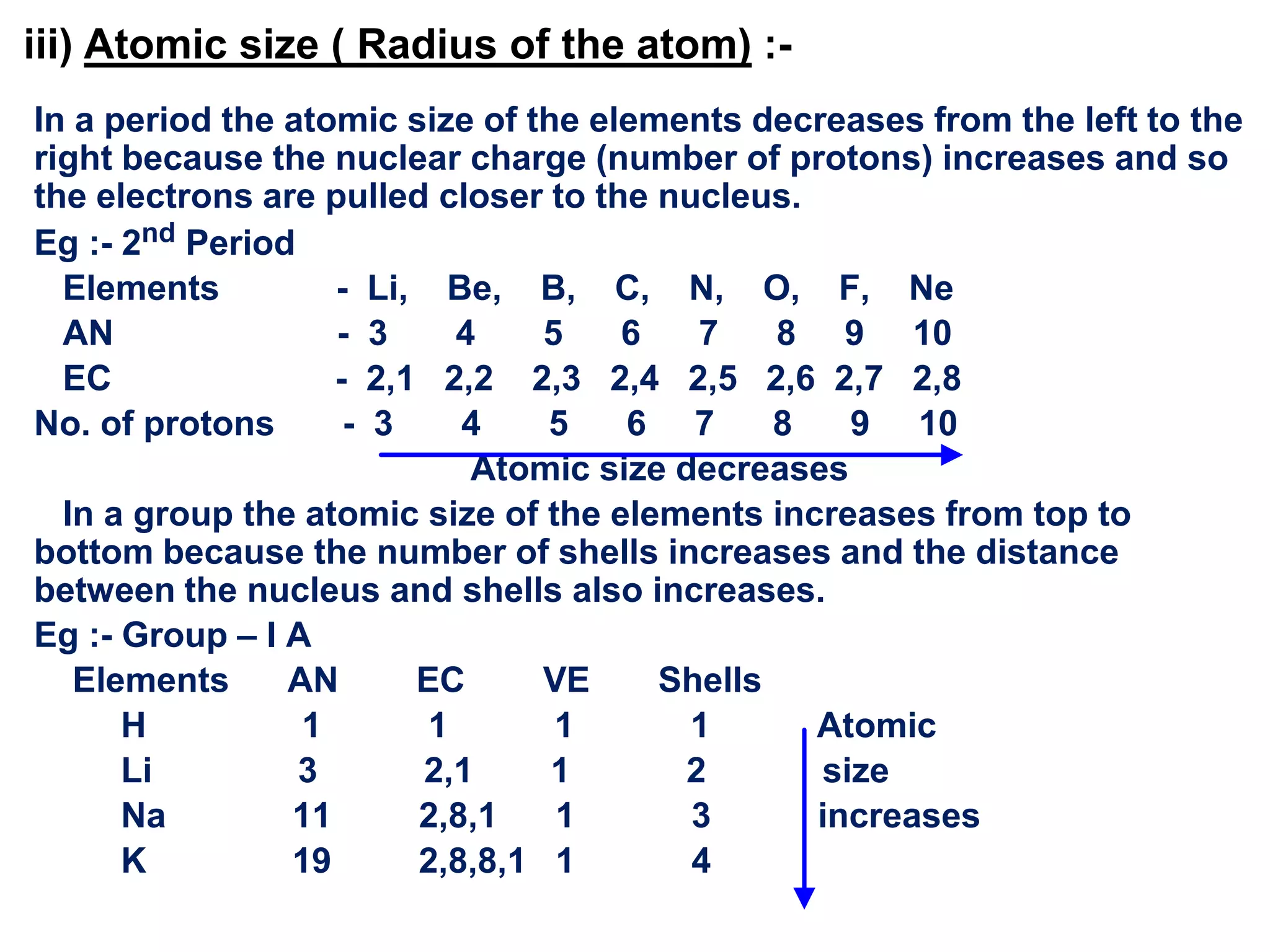
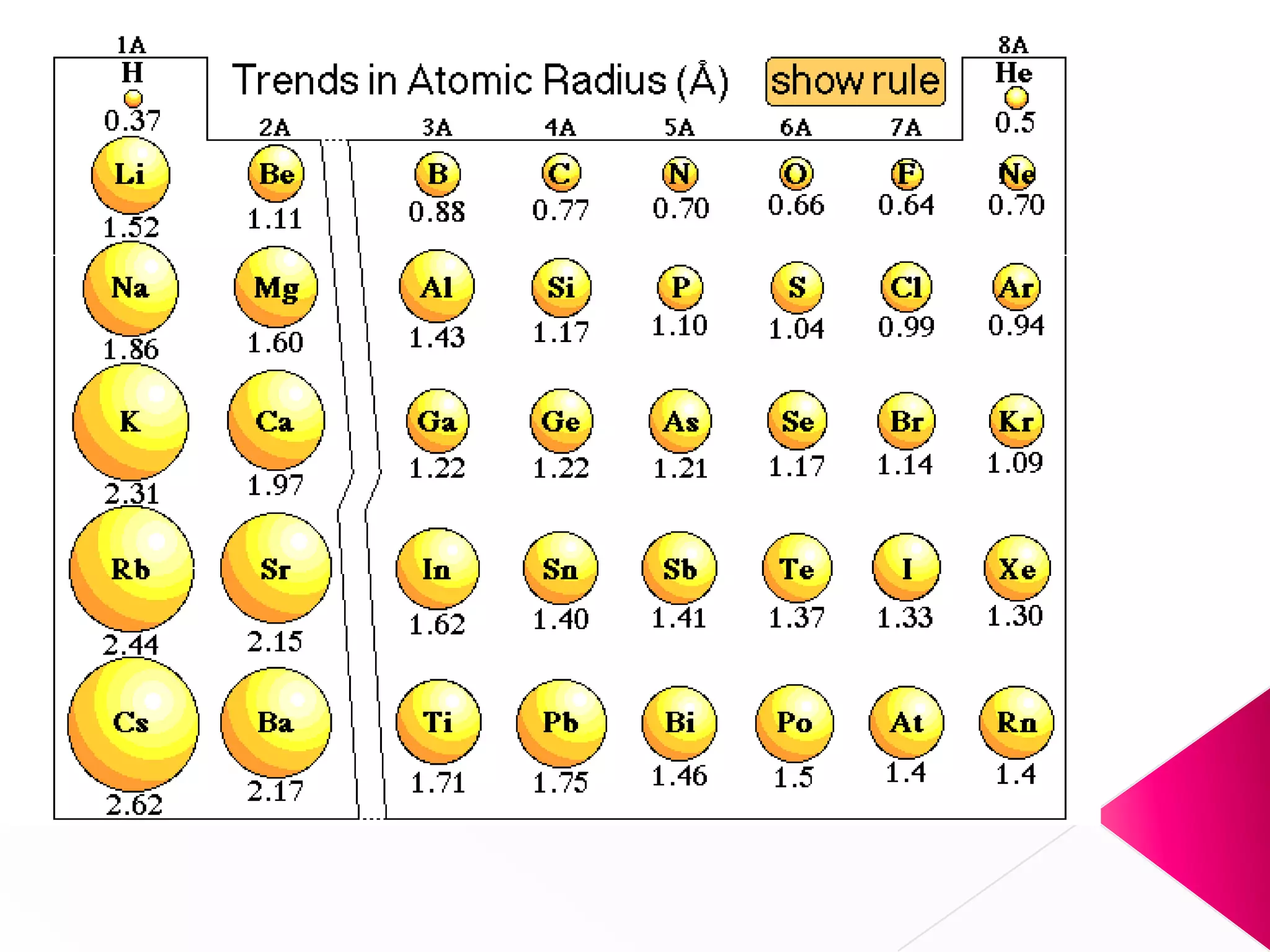
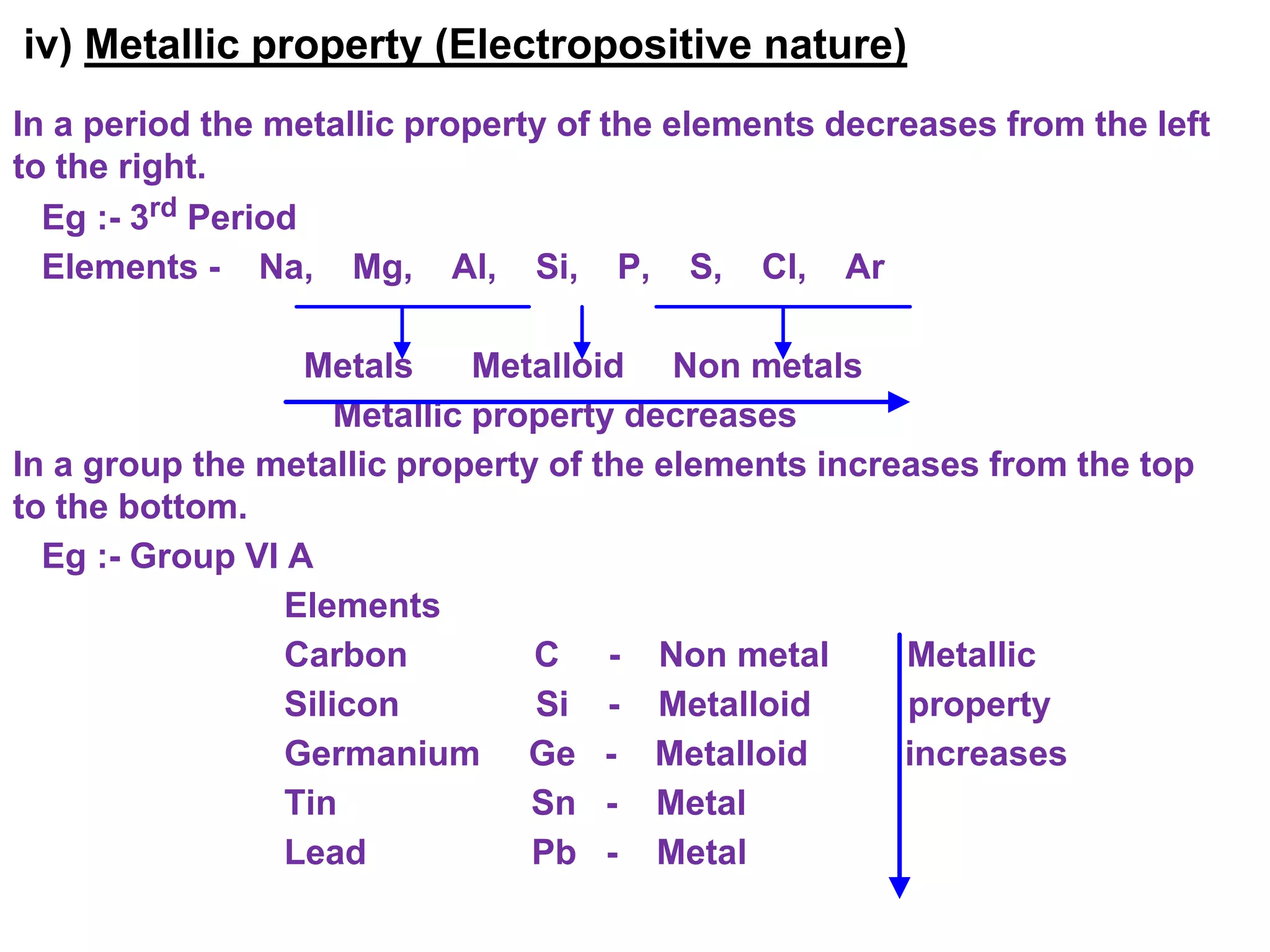
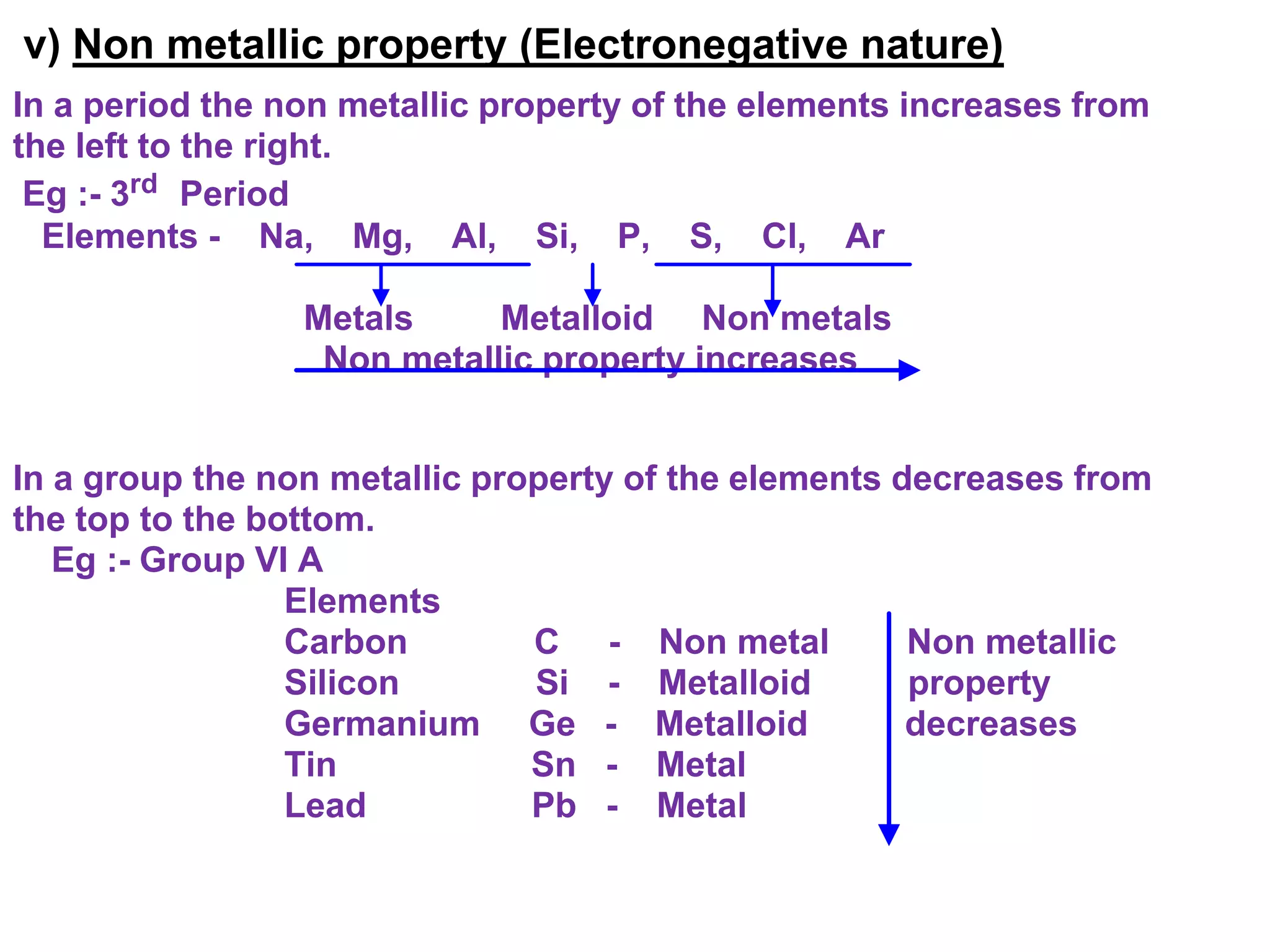
The document discusses the periodic classification of elements. It describes early attempts at classification including grouping elements as metals and non-metals. Dobereiner classified elements into triads based on atomic mass. Newlands arranged elements in order of increasing atomic mass in octaves but this classification had defects. Mendeleev arranged elements in order of atomic mass and predicted properties of undiscovered elements, establishing the basis of the modern periodic table. The modern periodic table arranges elements by atomic number and groups elements with similar properties and valence electrons.