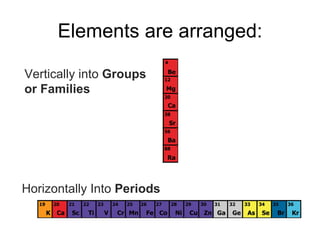
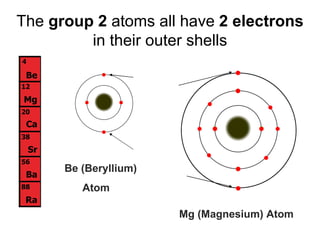
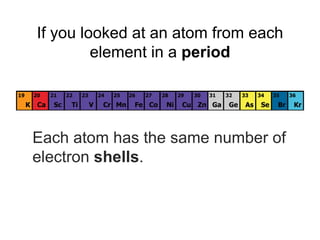
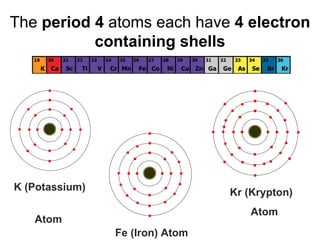
The document describes the classification of elements on the periodic table. Elements are arranged vertically into groups based on their valence electrons and horizontally into periods based on their atomic number. Metals are generally found on the left side of the periodic table, are shiny, good conductors, malleable, and ductile. Nonmetals are generally found on the right side, can exist as solids, liquids or gases, are typically dull and brittle, and poor conductors. Metalloids border both sides and have properties of both metals and nonmetals.