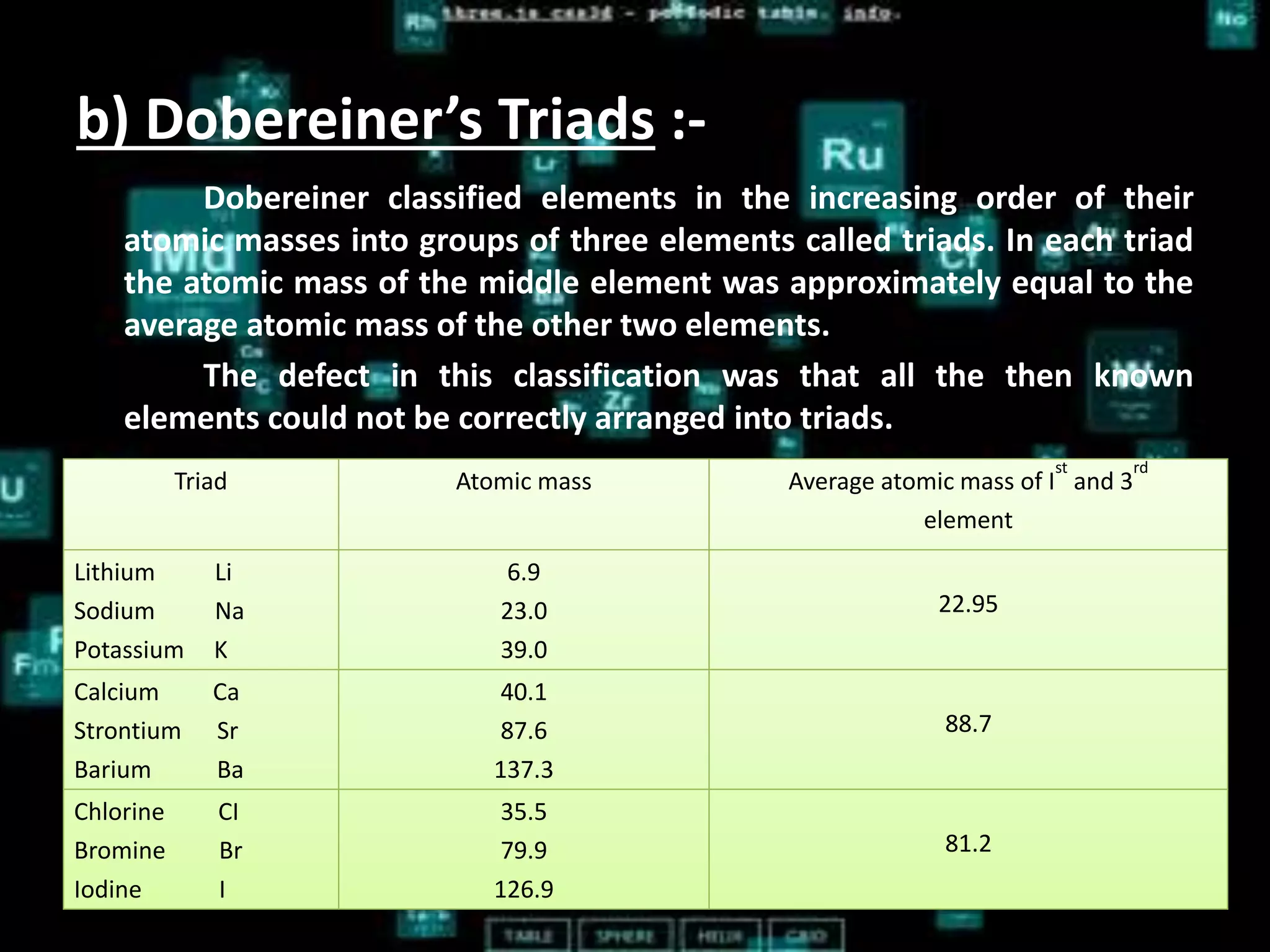
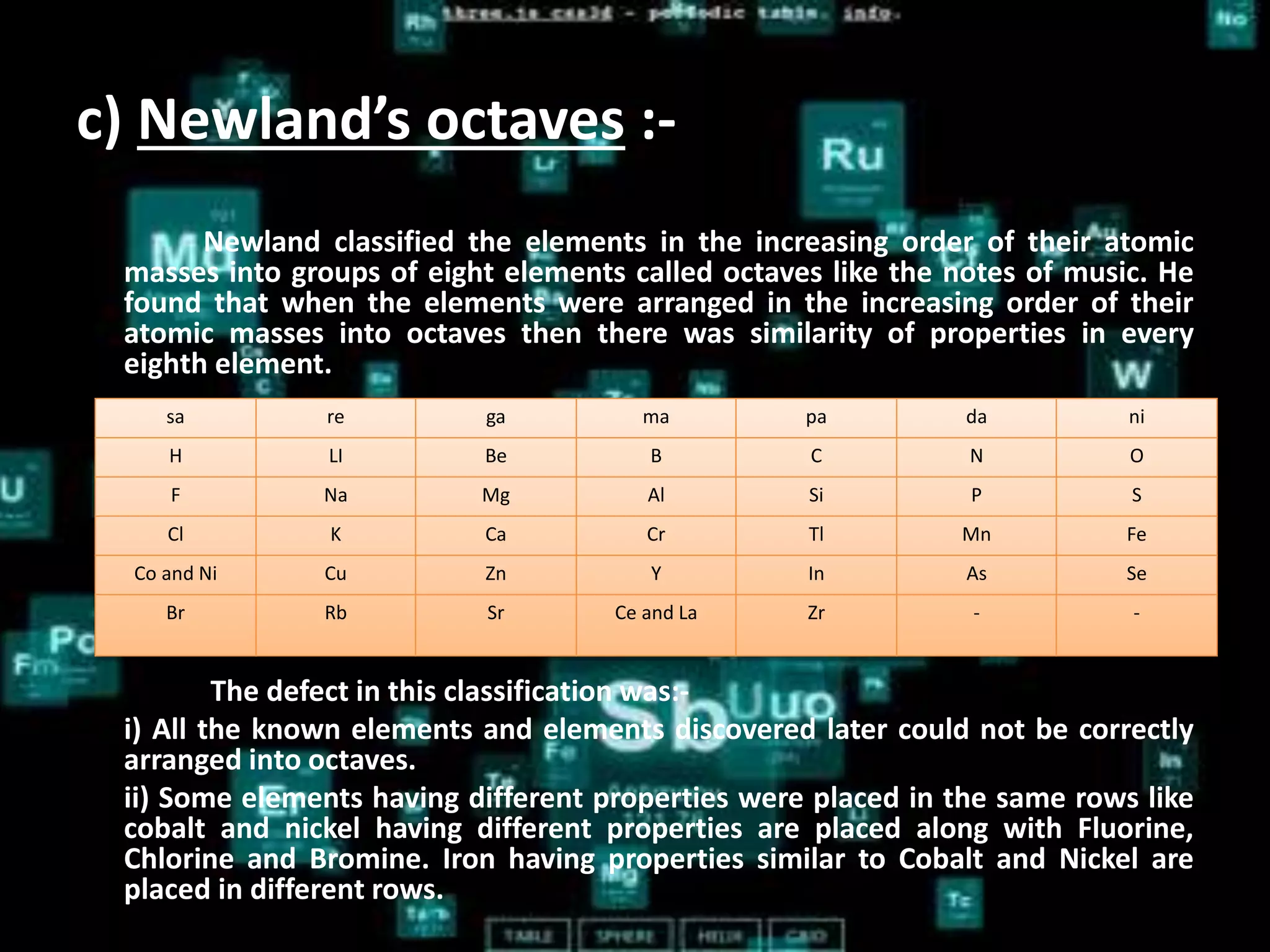
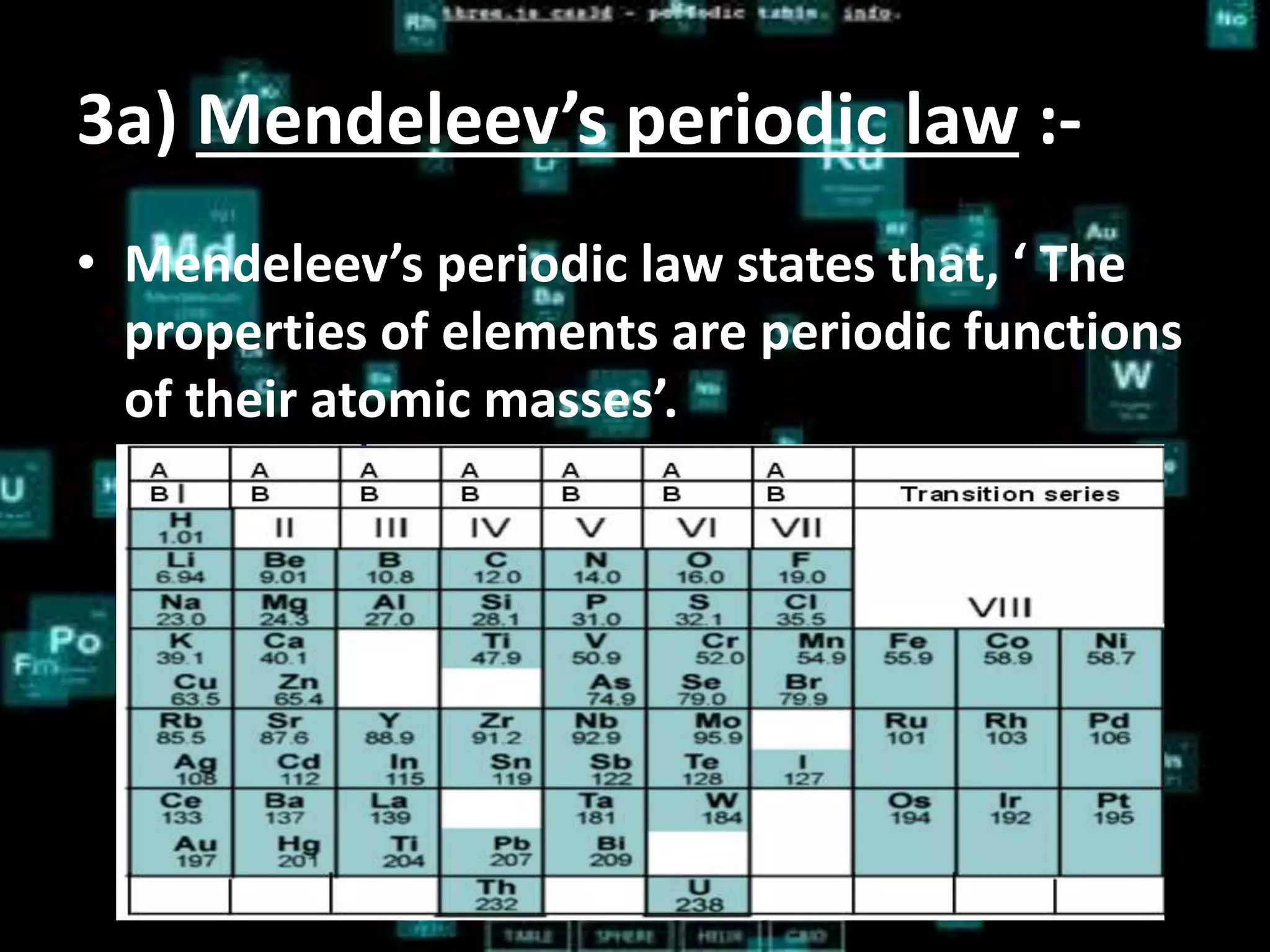
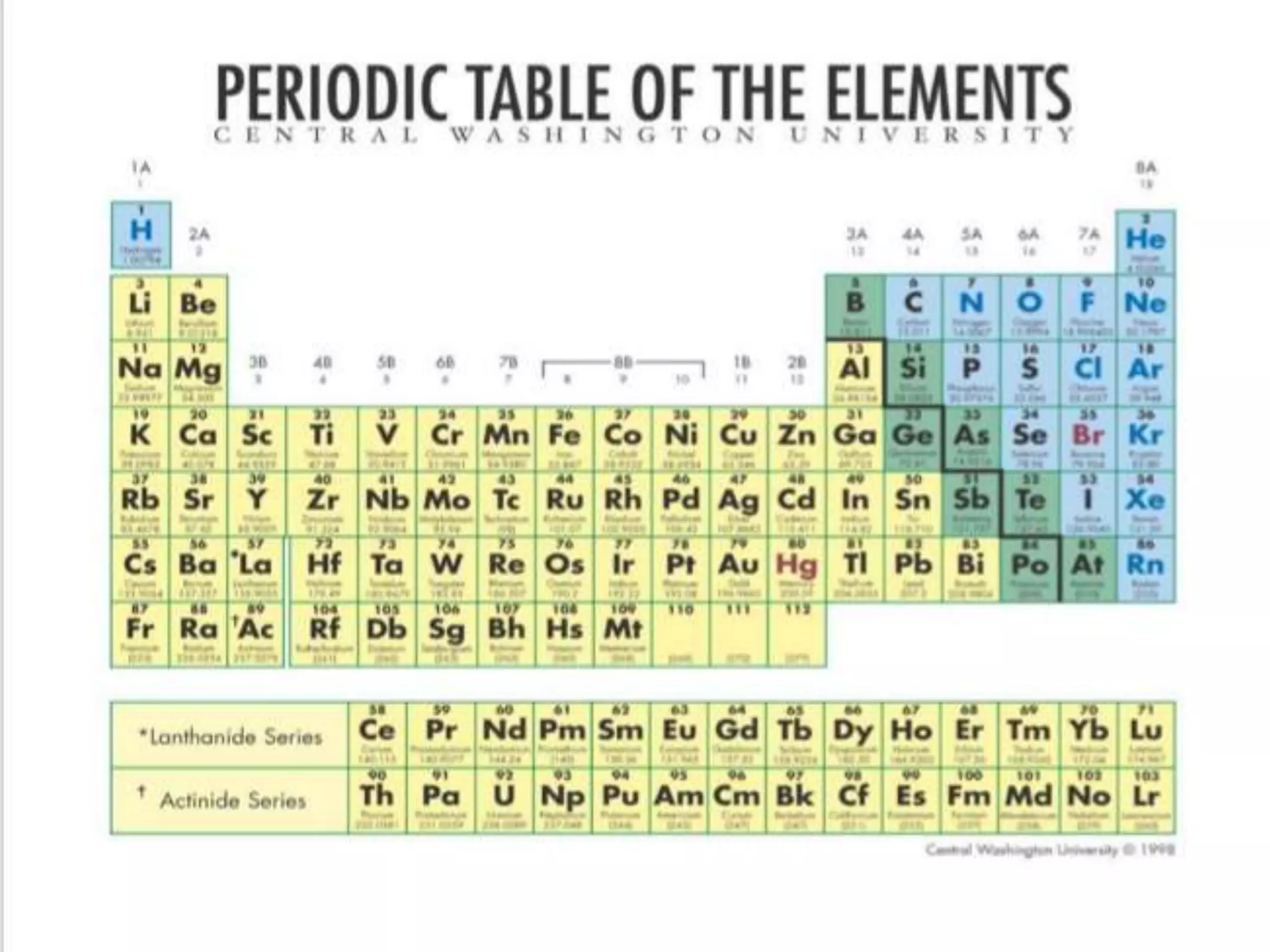
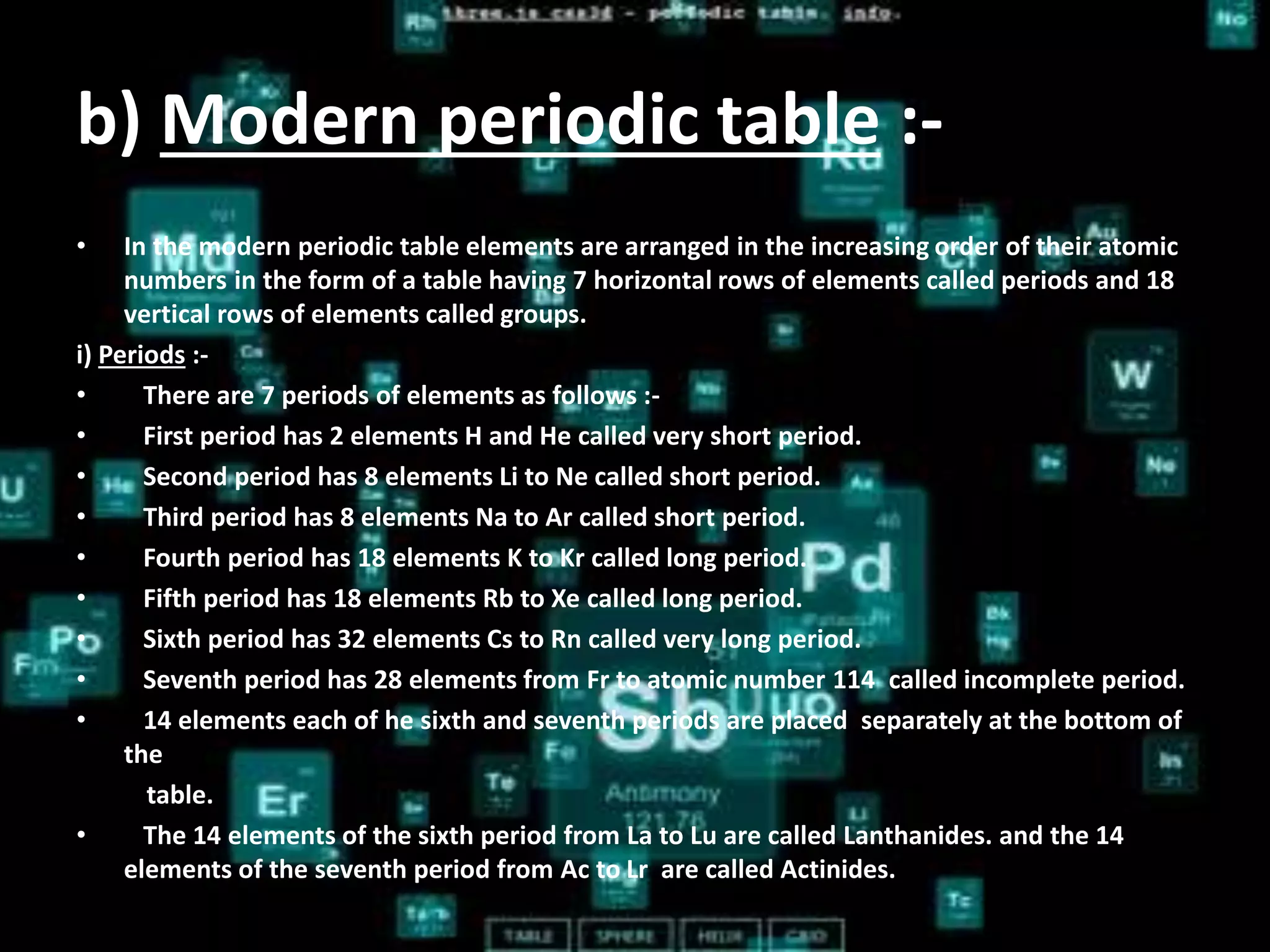
The document discusses the periodic classification of elements. It describes early attempts at classification by Dobereiner, Newlands, and Mendeleev. Mendeleev organized the elements into a periodic table based on atomic mass and properties. The modern periodic table arranges elements by atomic number and has 7 periods and 18 groups. Properties like valence electrons, valency, and atomic size are periodic within periods and groups based on the element's position in the table.