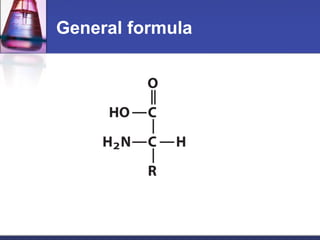
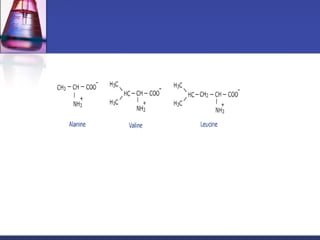
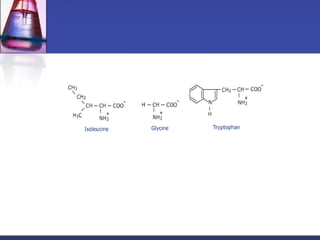
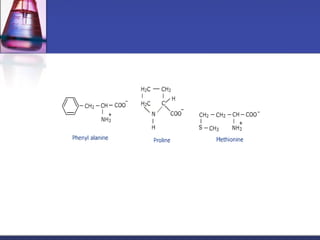
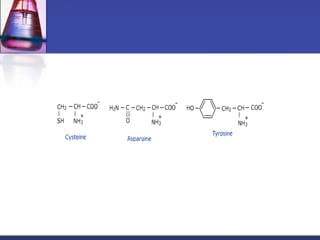
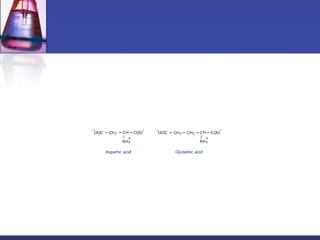
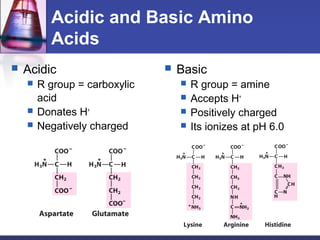
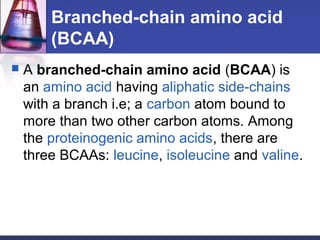
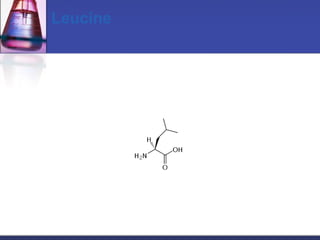
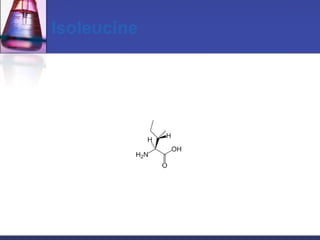
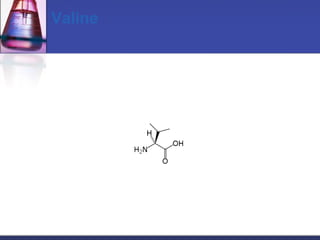
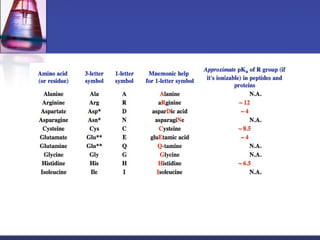
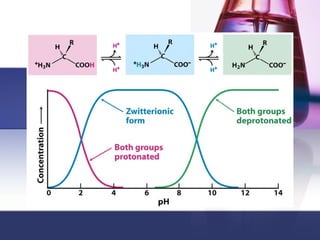
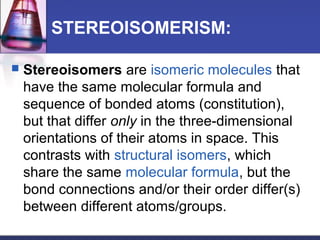
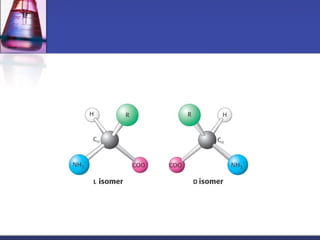
This document discusses amino acids, which are the building blocks of proteins. It defines amino acids as molecules containing both carboxyl and amine groups. There are over 300 amino acids found in nature, but only 22 are used as the standard building blocks in proteins. These standard amino acids differ in the side chain (R group) attached to their alpha carbon. Amino acids join together via peptide bonds to form protein chains. Proteins are essential to all living organisms and are formed through the process of translation.