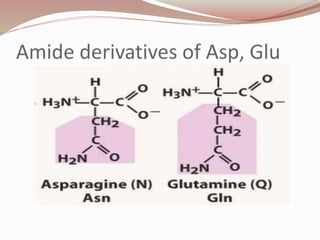
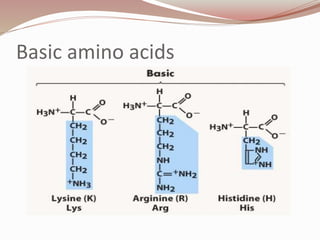
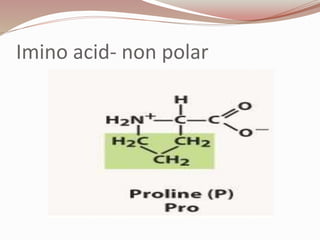
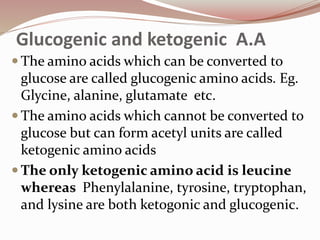
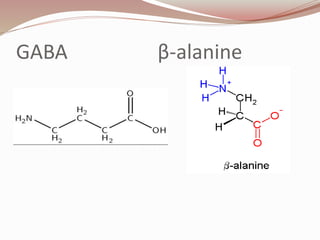
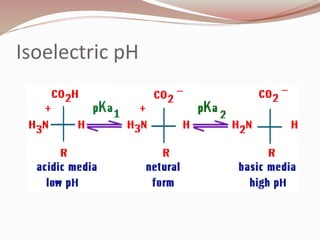
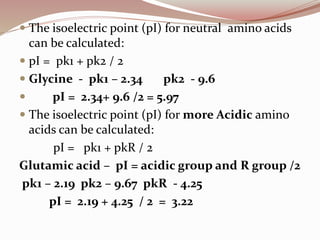
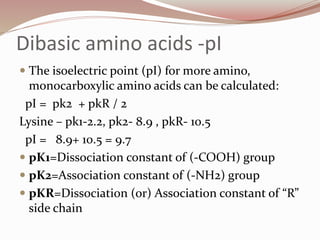
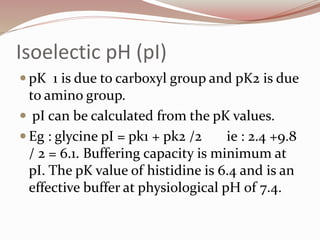
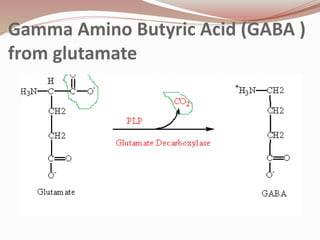
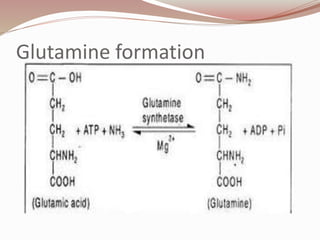
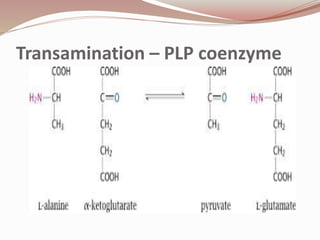
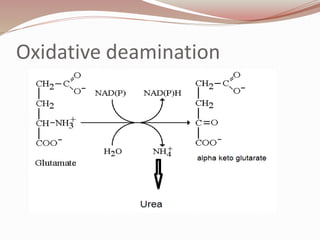
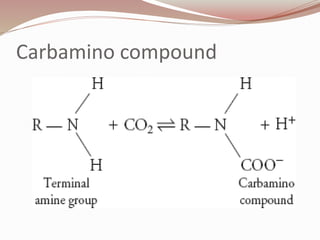
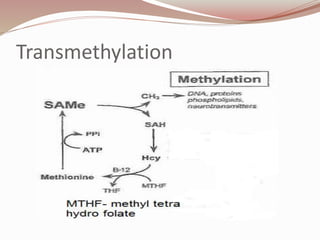
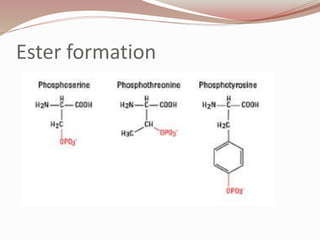
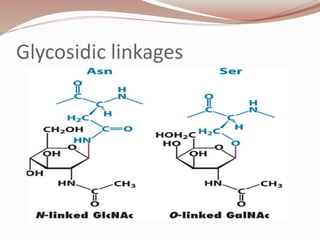
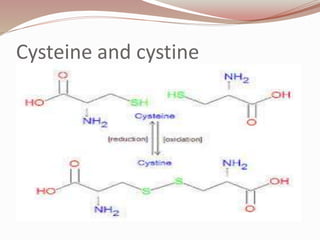
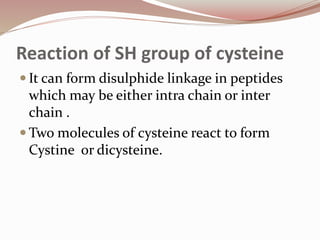
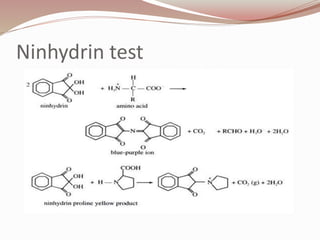
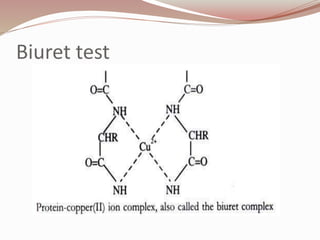
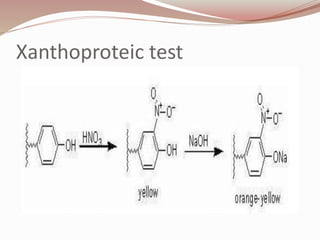
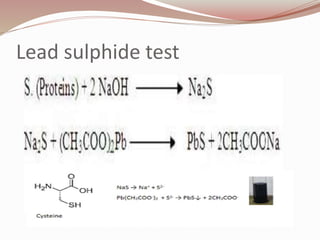
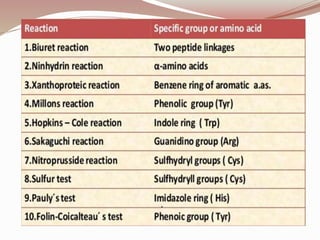
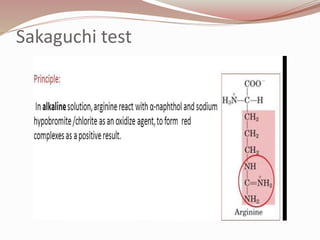
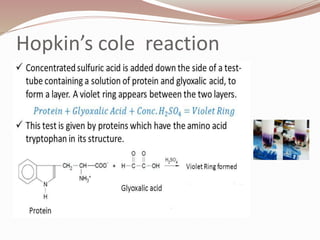
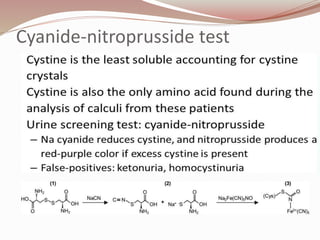
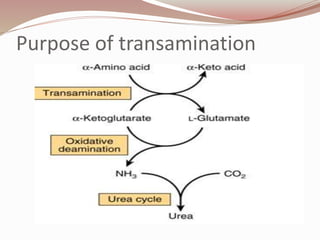
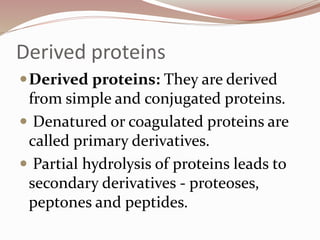
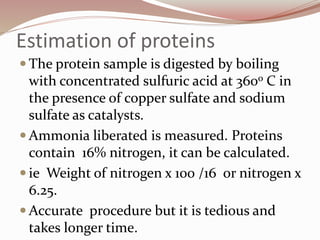
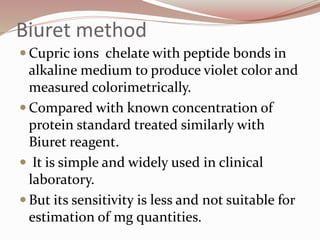
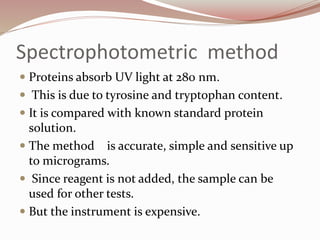
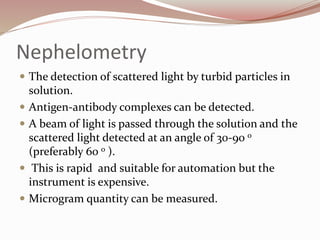
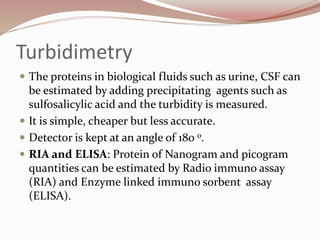
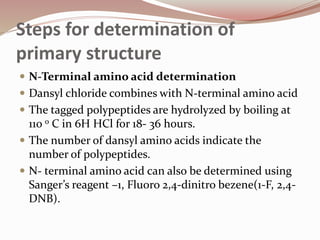
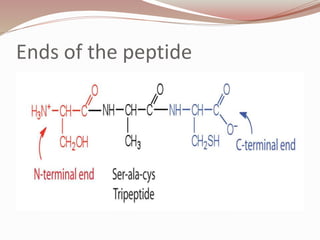
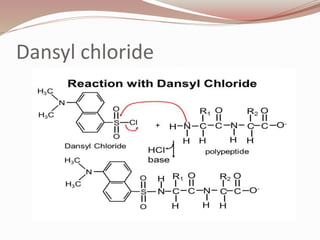
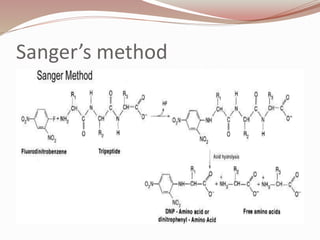
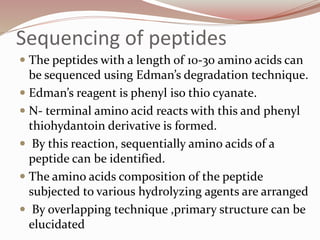
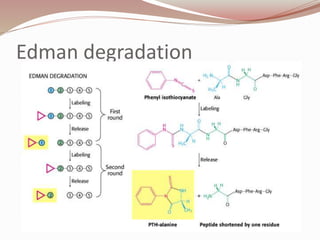
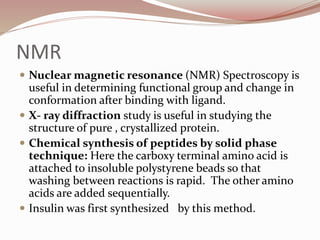
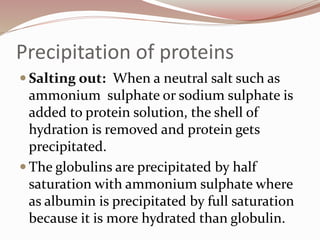
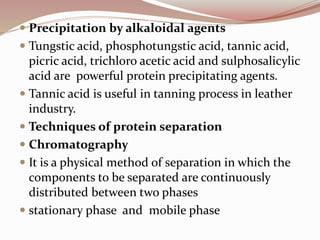
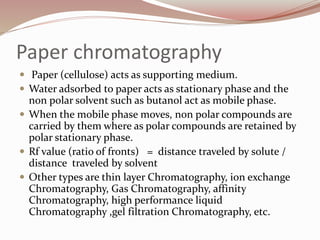
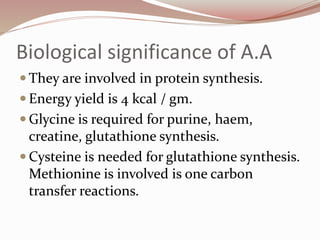
The document discusses the structure and properties of proteins and amino acids. Some key points:
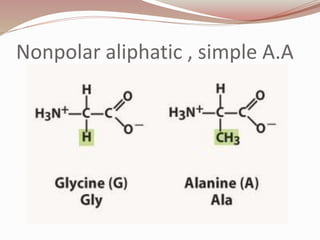
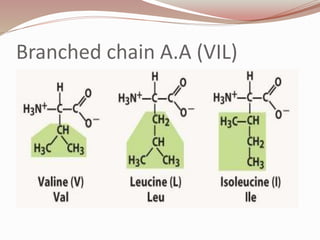
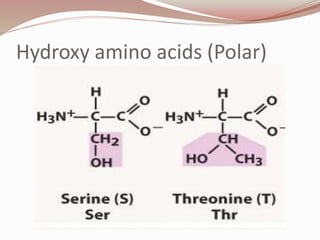
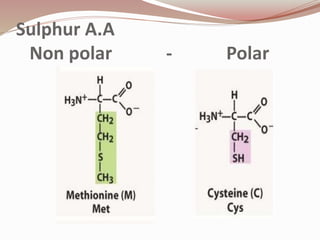
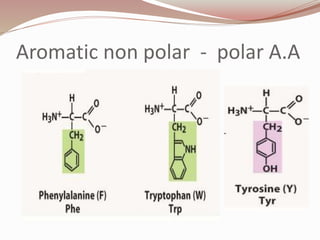
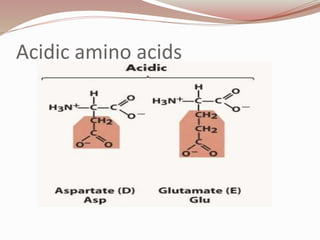
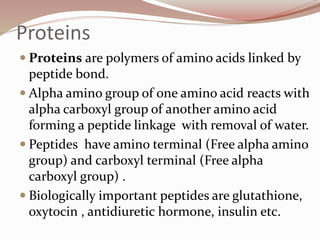
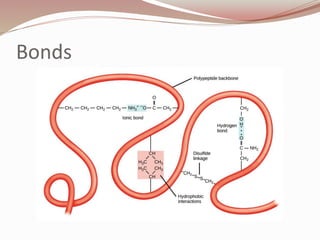
- Proteins are polymers of amino acids linked by peptide bonds. There are 20 standard amino acids that vary in properties based on their side chains.
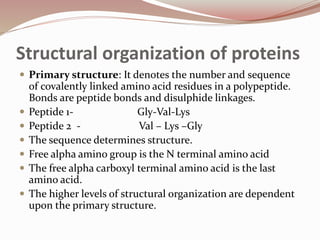
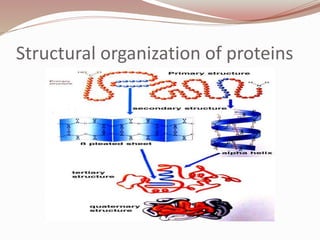
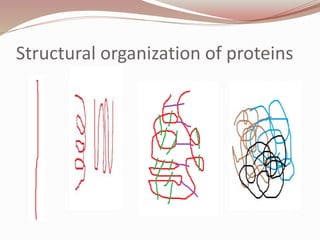
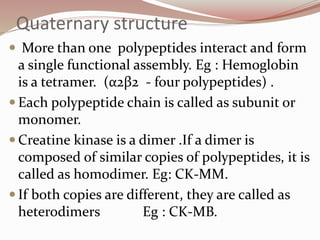
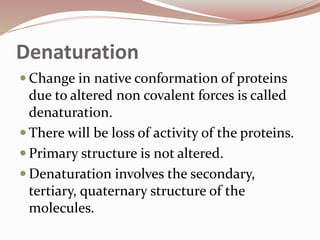
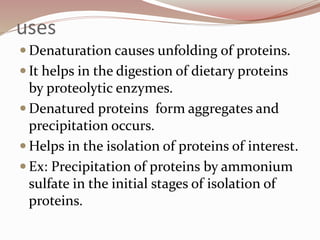
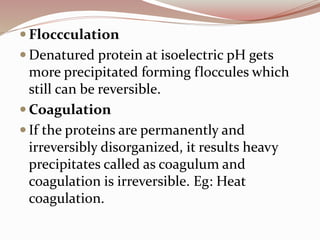
- Proteins have primary, secondary, tertiary, and quaternary levels of structure determined by amino acid sequence and interactions.
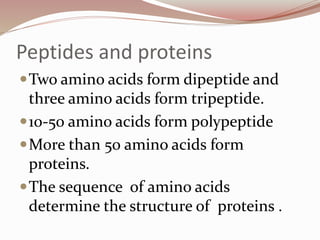
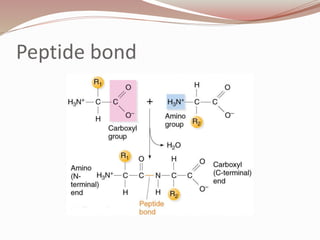
- Amino acids form peptides and proteins through condensation reactions between carboxyl and amino groups, forming peptide bonds.
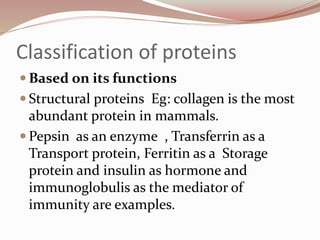
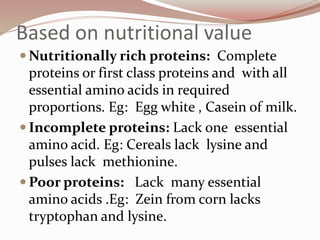
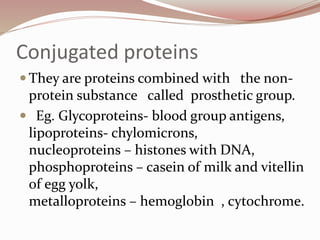
- Proteins can be classified based on function, shape, nutritional value, and composition. Structural proteins like collagen resist digestion while globular proteins are soluble.