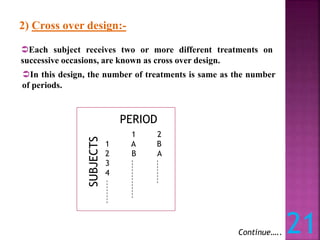
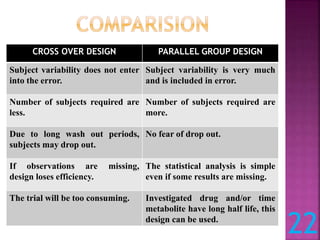
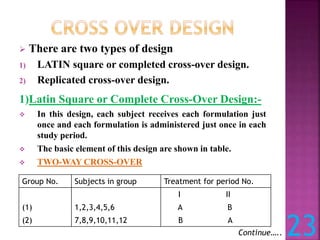
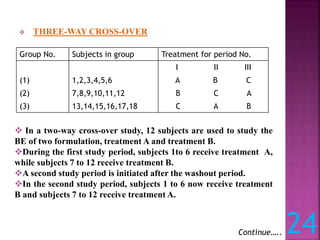
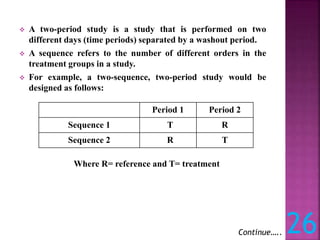
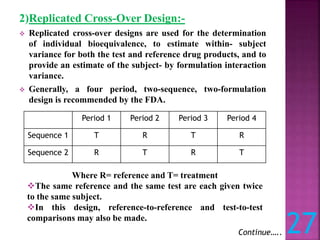
This document discusses bioavailability and bioequivalence studies. It defines bioavailability as the amount of drug that reaches systemic circulation in an unchanged form. Bioequivalence studies compare the test and reference formulations to show they have the same rate and extent of absorption. The document outlines study design considerations and parameters assessed, including Cmax, Tmax, and AUC from plasma concentration data. It also discusses single and multiple dose studies, as well as parallel and crossover study designs used in bioequivalence testing.






![[1] PLASMA DRUG CONCENTRATION DATA:-
Measurement of drug concentrations in blood, plasma or
serum after drug administration is the most direct and
objective way to determine systemic drug bioavailability.
tmax:- The time of peak plasma concentration tmax .
Time required to maximum drug concentration after drug
administration.
At tmax peak drug absorption occur and rate of drug
absorption is equal to rate of drug elimination.
Drug absorption still continues after tmax is reached at slower
rate.
10
Continue…..](https://image.slidesharecdn.com/bioavailabilitybioequivalence-230809071109-34abc317/85/BIO-AVAILABILITY-Bio-equivalence-pptx-12-320.jpg)

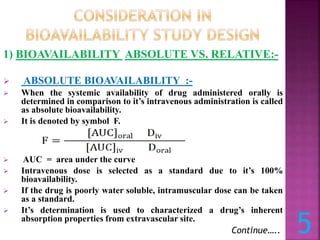
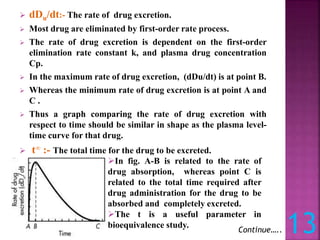
![[2] URINARY DRUG EXCRETION DATA:-
Urinary drug excretion data is an indirect method for
estimating bioavailability.
In addition, timely urine sample must be collected and total
amount of urinary drug excretion must be obtained.
Du:- The cumulative amount of drug excreted in the urine,
Du is directly related to the total amount of drug absorbed.
Urine sample are collected after administration of drug
product.
Each urine sample is analysed for free drug using specific
assay.
12
Continue…..
The relationship between cumulative
amount of drug excreted in urine and
plasma level – time curve is shown in
figure.
When drug is completely eliminated at
point C, the plasma concentration
approaches zero, and maximum amount
of drug excreted in urine, Du is
obtained.](https://image.slidesharecdn.com/bioavailabilitybioequivalence-230809071109-34abc317/85/BIO-AVAILABILITY-Bio-equivalence-pptx-14-320.jpg)
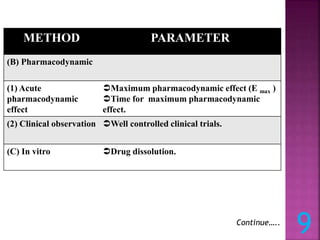
![[3] ACUTE PHARMACODYNAMIC EFFECT:-
When bioavailability measurement by pharmacodynamic method
is difficult, inaccurate or non reproducible.
Acute pharmacologic effect such as change in ECG or EEG
reading etc. is related to the time course of given drug.
An acute pharmacodynamic effect such as effect on forced
expiratory volume[FEV] or skin blanching can be use as an index of
drug bioavailability.
The acute pharmacodynamic effect is measured over period of
time after administration of drug product.
USE:
Acute pharmacodynamic effect determine bioavailability
generally requires demonstration of dose response curve.
Bioavailability is determined by characterization of dose response
curve.
The use of pharmacodynamic endpoint for the determination of
bioavailability and bioequivalence is much more variable than the
measurement of plasma or urine drug concentration.
Continue….. 14](https://image.slidesharecdn.com/bioavailabilitybioequivalence-230809071109-34abc317/85/BIO-AVAILABILITY-Bio-equivalence-pptx-16-320.jpg)
![15
[4] IN VITRO STUDIES:-
Drug dissolution studies may under certain conditions give an
indication of drug bioavailability.
Ideally, the in vitro drug dissolution rate should correlate with in
vivo drug bioavailability.
Dissolution studies are often performed on several test
formulations of the same drug.
The test formulation that demonstrates the most rapid rate of
drug dissolution.
In vitro will generally have the most rapid rate of drug
bioavailability in vivo.
The FDA may also use other in vitro approaches for establishing
bioequivalence.
For example, cholestyramine resin is a basic quaternary
ammonium action-exchange resin that is hydrophilic, insoluble in
water, and not absorbed in the gastrointestinal tract.
The bioequivalence of cholestyramine resin is performed by
equilibrium and kinetic binding studies of the resin to bile acid
salts.](https://image.slidesharecdn.com/bioavailabilitybioequivalence-230809071109-34abc317/85/BIO-AVAILABILITY-Bio-equivalence-pptx-17-320.jpg)