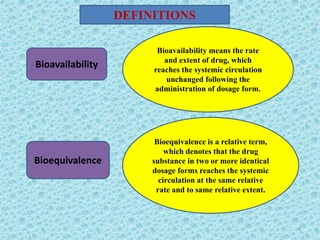
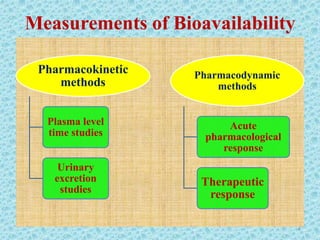
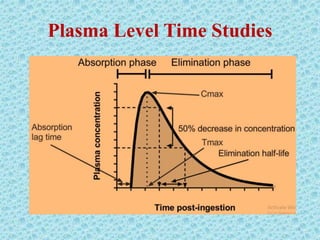
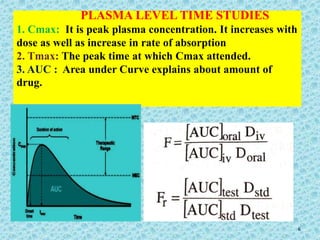
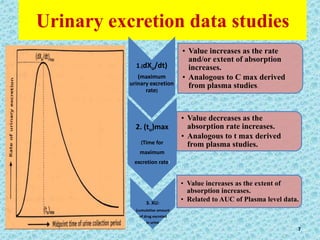
This document discusses measurements of bioavailability. It defines bioavailability and bioequivalence. There are two main methods to measure bioavailability - pharmacokinetic and pharmacodynamic. Pharmacokinetic methods include plasma level time studies and urinary excretion studies which measure parameters like Cmax, Tmax, and AUC from plasma data or urinary excretion rate and amount excreted from urine data. Pharmacodynamic methods include measuring acute pharmacological responses or therapeutic responses but have disadvantages like variable individual responses.