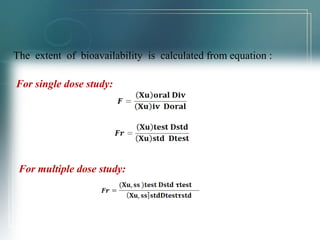
This document discusses bioavailability and methods for assessing it. Bioavailability refers to the rate and extent that an unchanged drug is absorbed and available at its site of action. It depends on pharmaceutical, patient, and route of administration factors. Methods for assessing bioavailability include plasma drug concentration-time profiles, urinary excretion studies, acute pharmacological response methods, and therapeutic response methods. The concept of equivalence is also introduced, which examines the relationship between different drug products.




![• When systemic availability of drug administered orally is
determined in comparison to its intravenous administration, is called
absolute bioavailability.
• Its determination is used to characterize a drug’s inherent
absorption properties from the extra vascular site..
AAbbssoolluuttee bbiiooaavvaaiillaabbiilliittyy == [[AAUUCC]]oorraall ((DDoossee))iivv
[[AAUUCC]]iivv ((DDoossee))oorraall](https://image.slidesharecdn.com/bioavailabilityppt-140930002953-phpapp01/85/Bioavailability-ppt-5-320.jpg)
![RReellaattiivvee BBiiooaavvaaiillaabbiilliittyy ((FFrr))
• When systemic availability of drug after oral administration is
compared with that of an oral standard of same drug (such as an
aqueous or non aqueous solution or suspension) it is referred as
relative bioavailability.
• It is used to characterize absorption of drug from its formulation.
RReellaattiivvee BBiiooaavvaaiillaabbiilliittyy == [[AAUUCC]]tteesstt ((DDoossee))ssttdd
[[AAUUCC]]ssttdd ((DDoossee))tteesstt](https://image.slidesharecdn.com/bioavailabilityppt-140930002953-phpapp01/85/Bioavailability-ppt-6-320.jpg)





![ AUC: Is the measurement of the extent of the drug bioavailability
It is the area under the drug plasma level-time curve from t =0 &
t = ∞, and is equal to the amount of unchanged drug reaching the
general circulation divided by the clearance.
[ AUC]0
∞ = ∫0
∞Cpdt
[ AUC]0
∞ = FD0 = FDO/ kVD
Clearance](https://image.slidesharecdn.com/bioavailabilityppt-140930002953-phpapp01/85/Bioavailability-ppt-12-320.jpg)