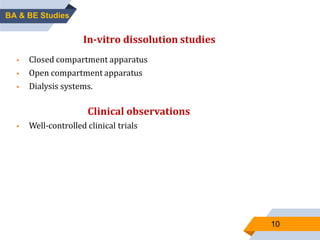
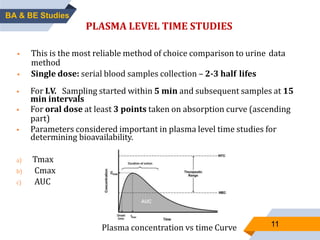
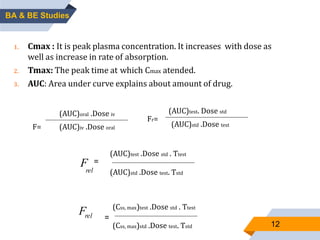
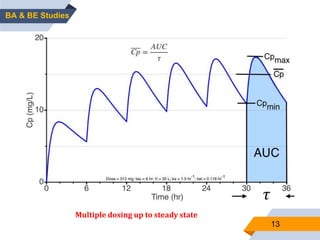
1. The document discusses bioavailability and bioequivalence studies, which are important for determining the rate and extent of drug absorption from different formulations.
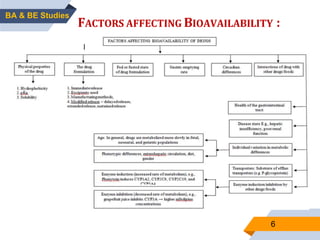
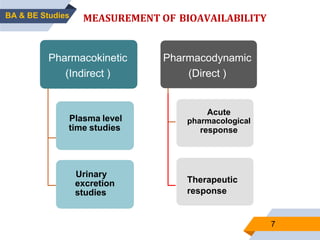
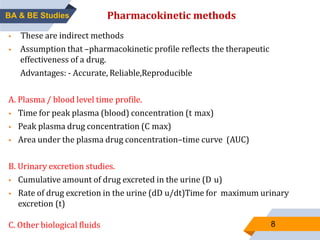
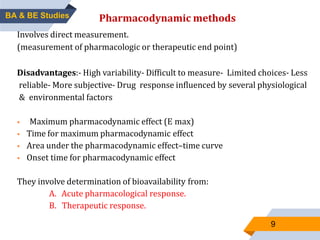
2. Key aspects of bioavailability studies include absolute and relative bioavailability, factors affecting bioavailability, and methods of measuring bioavailability through pharmacokinetic and pharmacodynamic approaches.
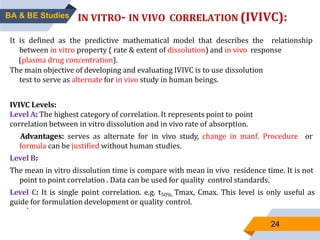
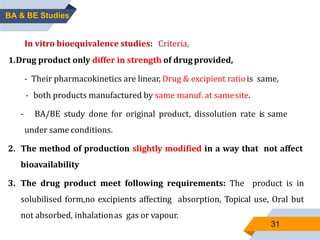
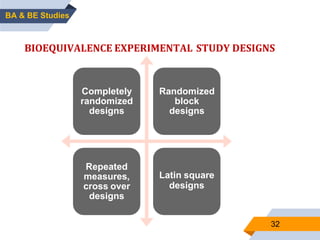
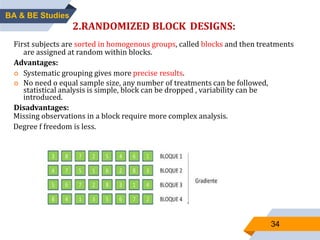
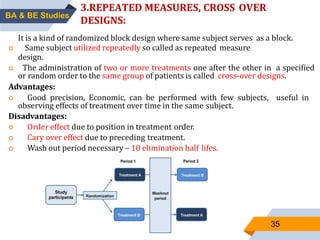
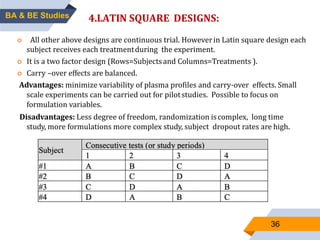
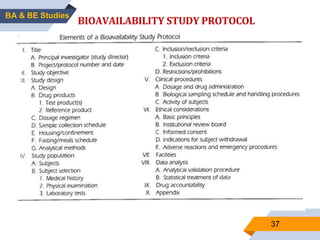
3. Bioequivalence studies aim to show that two products have identical plasma concentration time profiles and are therefore interchangeable. Different study designs like randomized and crossover designs are used to evaluate bioequivalence.

![4
ABSOLUTE BIOAVAILABILITY:
The systemic availability of a drug administered orally is determined in
comparison to its iv administration.
Characterization of a drug's absorption properties from thee.v. site.
Intravenous dose is selected as a standard due to its100% bioavailability
If the drug is poorly water soluble, intramuscular dose canbe taken as
standard.
Its determination is used to characterize a drug’s inherent absorption
properties from extravascularsite.
Absolute bioavailability (F):
Dose (iv) x [AUC] (oral)
F = ------------------------------- X 100
Dose (oral) x [AUC] (iv)
BA & BE Studies
Absolute bioavailability (F):](https://image.slidesharecdn.com/bioavailabilityandbioequivalanestudies-200415061422/85/Bioavailability-and-bioequivalane-studies-4-320.jpg)
![5
RELATIVE BIOAVAILABILITY:
The availability of a drug product as compared to another dosage form or
product of the same drug given in thesame dose.
Characterization of absorption of a drug fromits formulation.
The standard is a pure drug evaluated in a crossoverstudy. Its determination
is used to characterize absorption ofdrug from its formulation.
Both F and Fr are expressed as percentage
Relative bioavailability (Frel)
BA & BE Studies
Relative bioavailability (Fr):
Dose (std) x [AUC] (test)
Fr = ------------------------------- X 100
Dose (test) x [AUC] (std)](https://image.slidesharecdn.com/bioavailabilityandbioequivalanestudies-200415061422/85/Bioavailability-and-bioequivalane-studies-5-320.jpg)