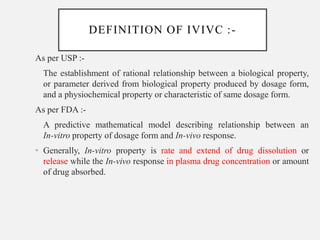
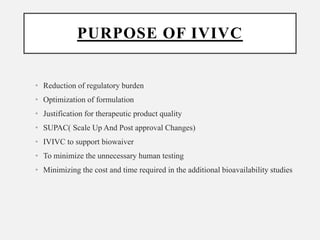
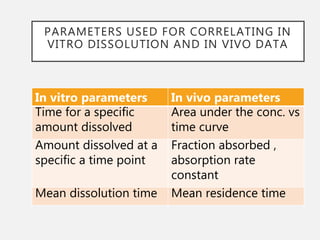
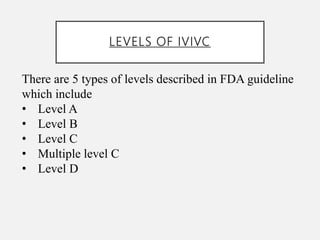
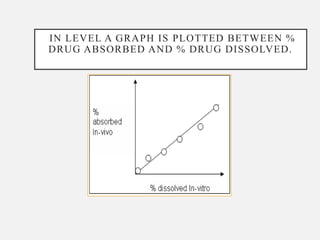
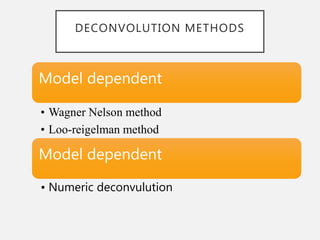
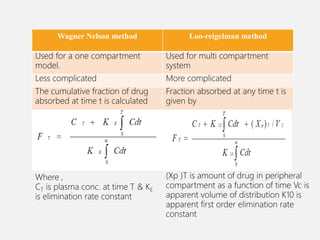
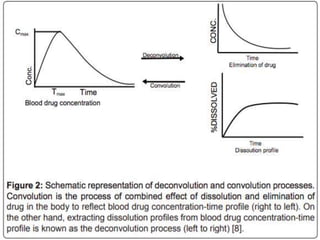
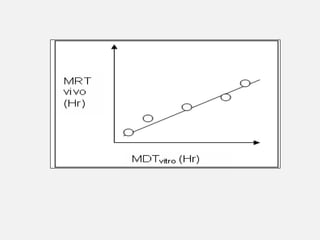
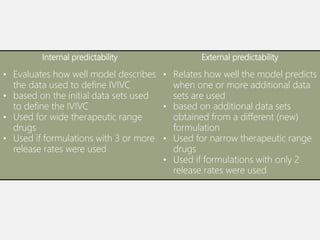
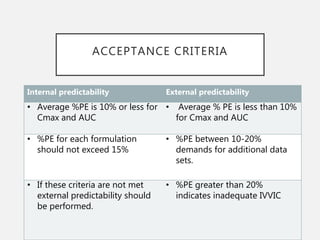
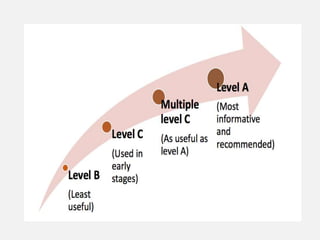
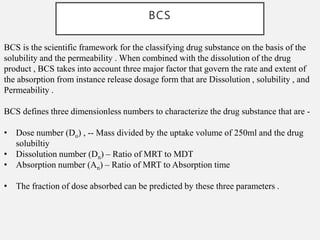
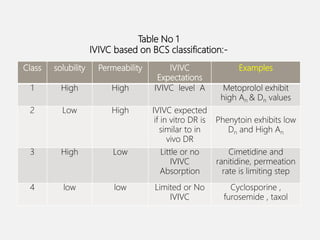
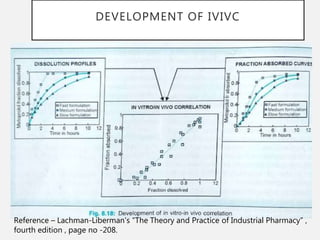
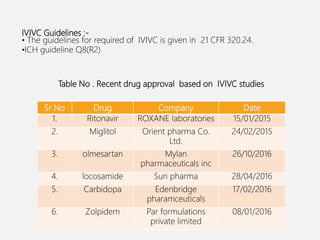
In vitro–in vivo correlation (IVIVC) is vital in drug development, enabling dosage form optimization while minimizing human testing and costs. It establishes a relationship between in vitro dissolution properties and in vivo responses, categorized into levels A, B, C, and D, each with varying predictive strength. The FDA emphasizes IVIVC's role in facilitating biowaivers and ensuring therapeutic product quality.