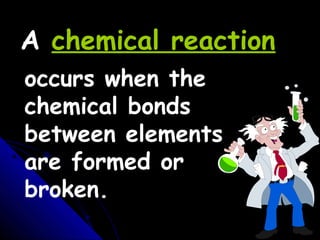
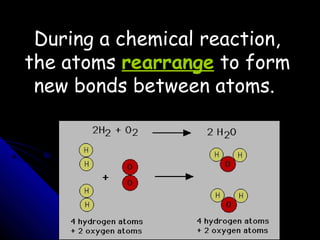
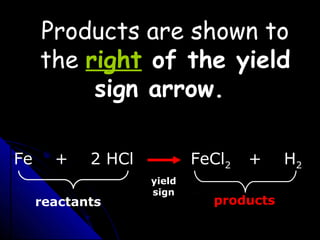
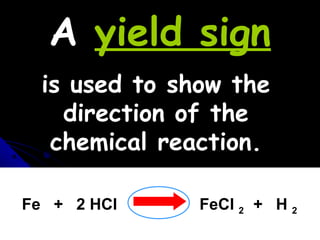
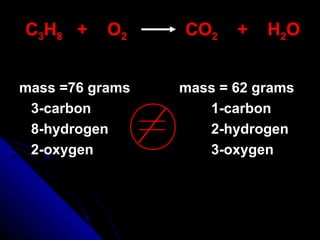
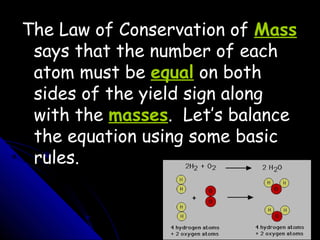
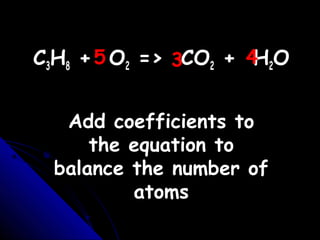
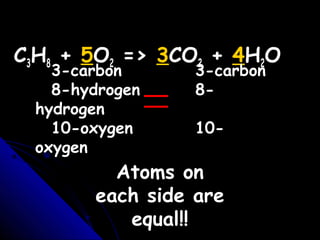This document discusses balancing chemical equations. It explains that a chemical equation uses symbols and formulas to represent a chemical reaction and must obey the law of conservation of mass. This law states that the number and type of atoms on the reactants side must equal the number and type on the products side. The document shows an example of an unbalanced equation and uses coefficients to balance the atoms, resulting in an equation that follows the law of conservation of mass.