Embed presentation
Downloaded 149 times
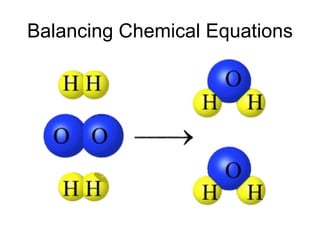
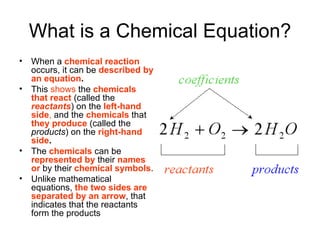
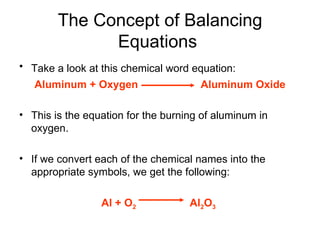
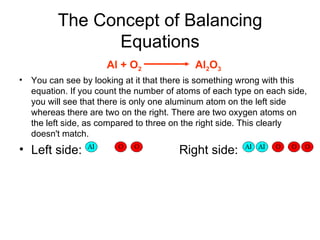
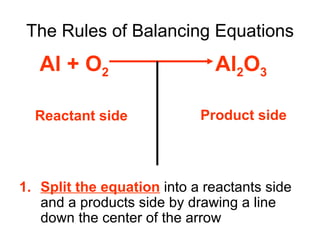
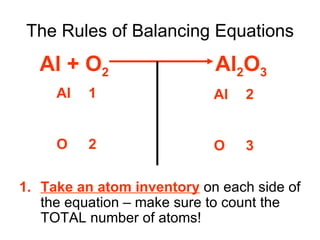
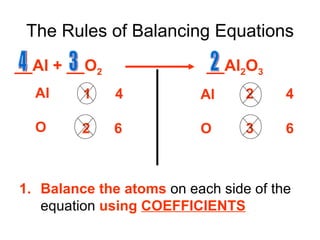
A chemical equation represents a chemical reaction, showing the reactants on the left and products on the right separated by an arrow. For the equation to be balanced, the number of atoms of each type must be equal on both sides. To balance an equation, you take an atom inventory, split the equation into reactants and products, then add coefficients in front of the chemical formulas to make the number of atoms equal on both sides.






