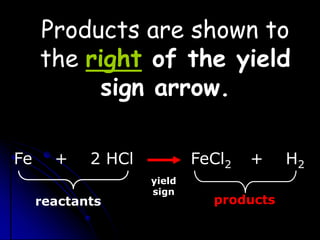
The document discusses balancing chemical equations by following the law of conservation of mass. It explains that a chemical equation uses symbols to represent a chemical reaction, with reactants on the left side of the yield sign and products on the right. For the equation to be balanced, the number of atoms of each element must be equal on both sides after coefficients are added. This ensures the mass is also conserved, as required by the law of conservation of mass. An example demonstrates balancing the equation for the combustion of propane by adding coefficients to match the number of carbon, hydrogen and oxygen atoms on each side.