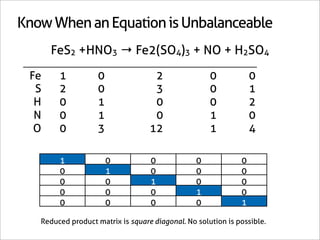
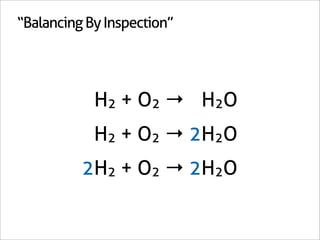
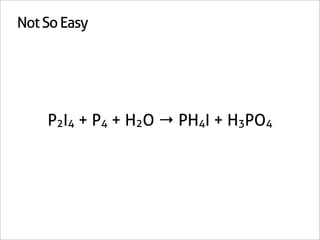
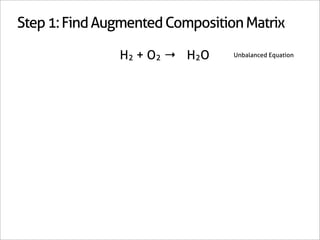
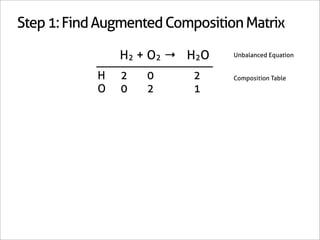
The document outlines a systematic method for balancing chemical equations, emphasizing a step-by-step approach that eliminates guesswork. It describes techniques such as obtaining an augmented composition matrix, inverting it, and assigning coefficients, applicable to both redox and non-redox reactions. The content includes examples and mentions an app that can assist in this balancing process.






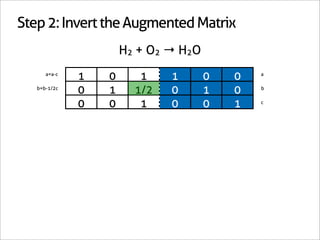
![Step 3: Assign Coefficients
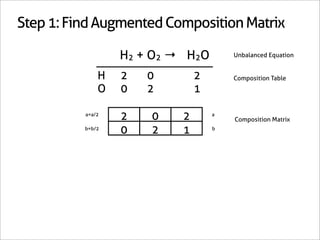
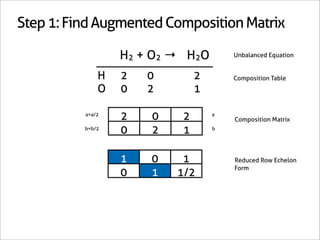
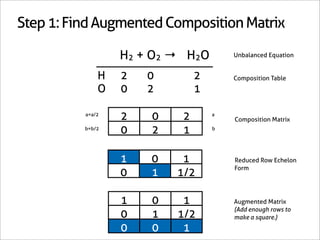
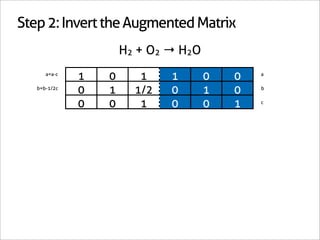
H2 + O2 → H2O
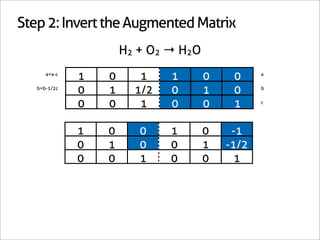
1 0 -1
0 1 -1/2 Matrix Inverse
0 0 1
[-1 -1/2 1] Null Space Vector Transpose](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-24-320.jpg)
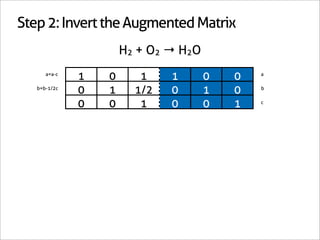
![Step 3: Assign Coefficients
H2 + O2 → H2O
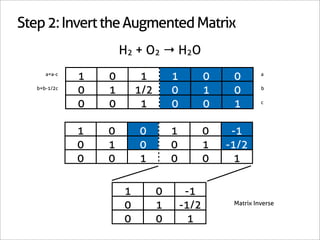
1 0 -1
0 1 -1/2 Matrix Inverse
0 0 1
[-1 -1/2 1] Null Space Vector Transpose
x2 removes fractions [-2 -1 2] Scaled Transpose
Negative: reactant
Positive: product](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-25-320.jpg)
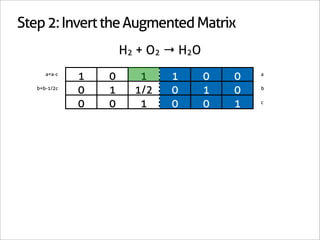
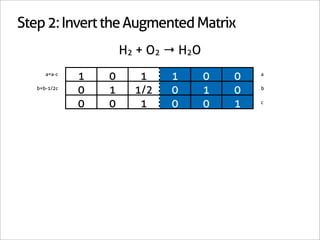
![Step 3: Assign Coefficients
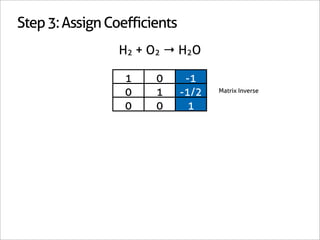
H2 + O2 → H2O
1 0 -1
0 1 -1/2 Matrix Inverse
0 0 1
[-1 -1/2 1] Null Space Vector Transpose
x2 removes fractions [-2 -1 2] Scaled Transpose
Negative: reactant
Positive: product
2H2 + 1O2 → 2H2O](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-26-320.jpg)

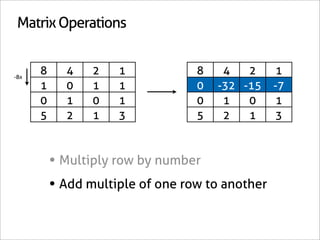
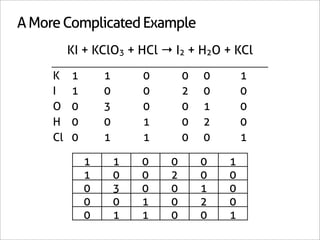
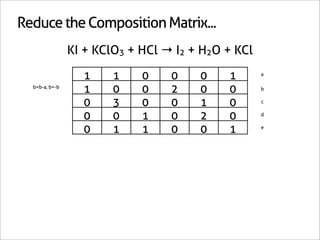
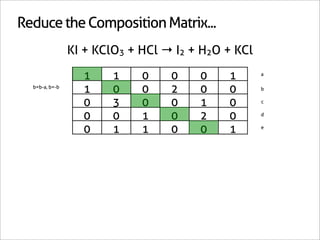
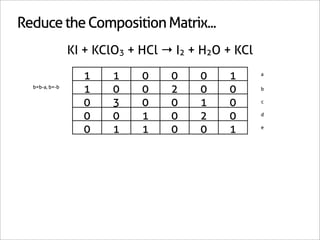
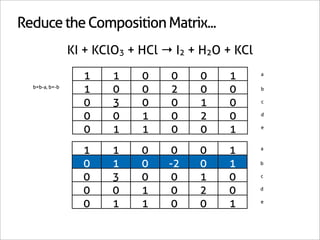
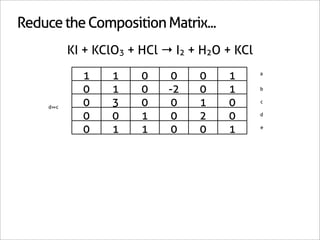
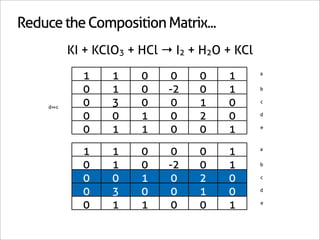
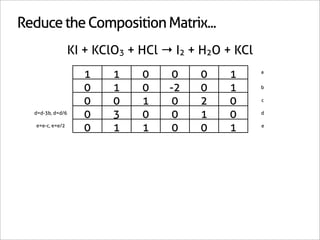
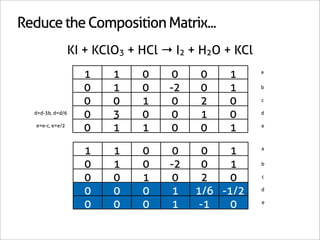
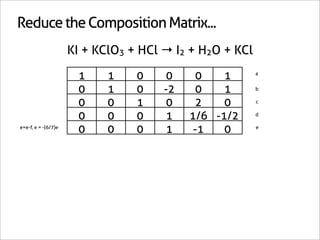
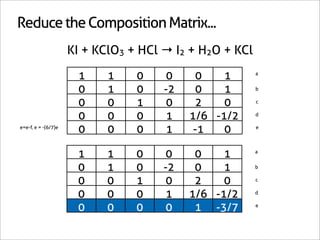
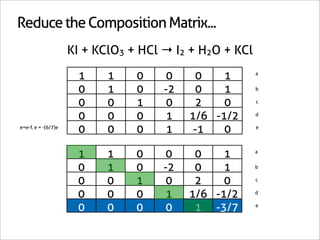
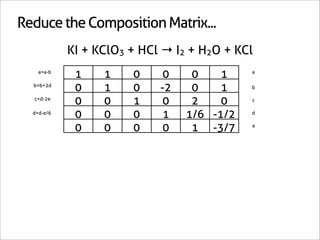
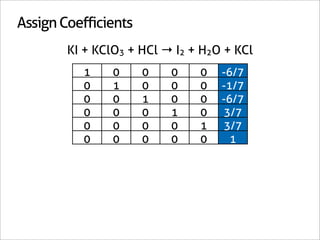
![Assign Coefficients
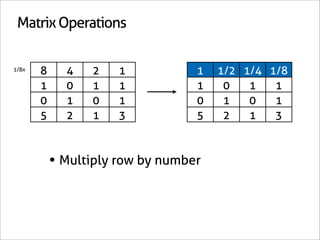
KI + KClO3 + HCl → I2 + H2O + KCl
1 0 0 0 0 -6/7
0 1 0 0 0 -1/7
0 0 1 0 0 -6/7
0 0 0 1 0 3/7
0 0 0 0 1 3/7
0 0 0 0 0 1
x7 [-6/7 -1/7 -6/7 3/7 3/7 1]
[-6 -1 -6 3 3 7]](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-50-320.jpg)
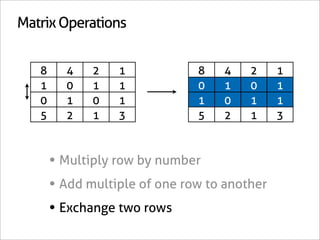
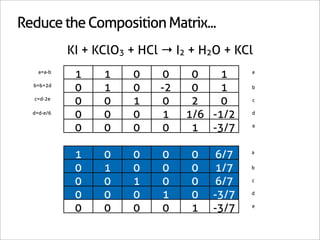
![Assign Coefficients
KI + KClO3 + HCl → I2 + H2O + KCl
1 0 0 0 0 -6/7
0 1 0 0 0 -1/7
0 0 1 0 0 -6/7
0 0 0 1 0 3/7
0 0 0 0 1 3/7
0 0 0 0 0 1
x7 [-6/7 -1/7 -6/7 3/7 3/7 1]
[-6 -1 -6 3 3 7]
6KI + 1KClO3 + 6HCl → 3I2 + 3H2O + 7KCl](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-51-320.jpg)
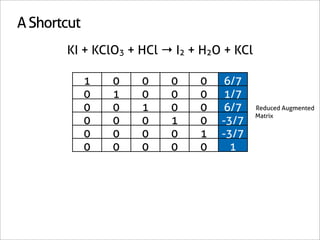
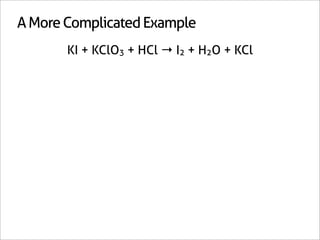
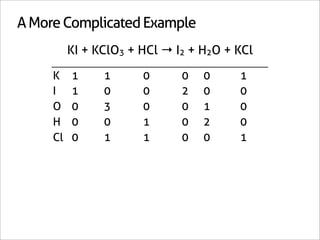
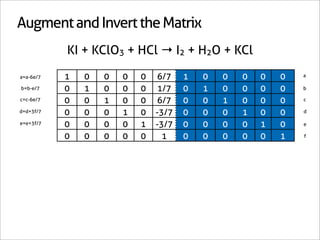
![A Shortcut
KI + KClO3 + HCl → I2 + H2O + KCl
1 0 0 0 0 6/7
0 1 0 0 0 1/7
0 0 1 0 0 6/7 Reduced Augmented
Matrix
0 0 0 1 0 -3/7
0 0 0 0 1 -3/7
0 0 0 0 0 1
[6/7 1/7 6/7 -3/7 -3/7 1]
x7 [6 1 6 3 3 7]](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-53-320.jpg)
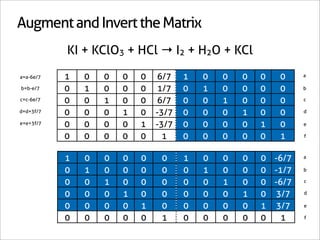
![A Shortcut
KI + KClO3 + HCl → I2 + H2O + KCl
1 0 0 0 0 6/7
0 1 0 0 0 1/7
0 0 1 0 0 6/7 Reduced Augmented
Matrix
0 0 0 1 0 -3/7
0 0 0 0 1 -3/7
0 0 0 0 0 1
[6/7 1/7 6/7 -3/7 -3/7 1]
x7 [6 1 6 3 3 7]
6KI + 1KClO3 + 6HCl → 3I2 + 3H2O + 7KCl](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-54-320.jpg)
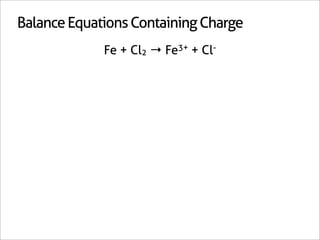
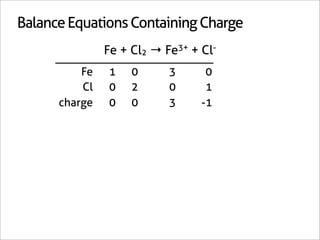
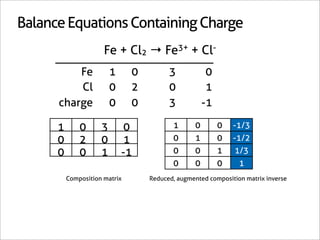
![Balance Equations Containing Charge
Fe + Cl2 → Fe3+ + Cl-
Fe 1 0 3 0
Cl 0 2 0 1
charge 0 0 3 -1
1 0 3 0 1 0 0 -1/3
0 2 0 1 0 1 0 -1/2
0 0 1 -1 0 0 1 1/3
0 0 0 1
Composition matrix Reduced, augmented composition matrix inverse
x7 [-1/3 -1/2 1/3 1] → [-2 -3 2 6]
2Fe + 3Cl2 → 2Fe3+ + 6Cl-](https://image.slidesharecdn.com/balance-chemical-equations-130219192520-phpapp01/85/How-to-Balance-Any-Chemical-Equation-58-320.jpg)