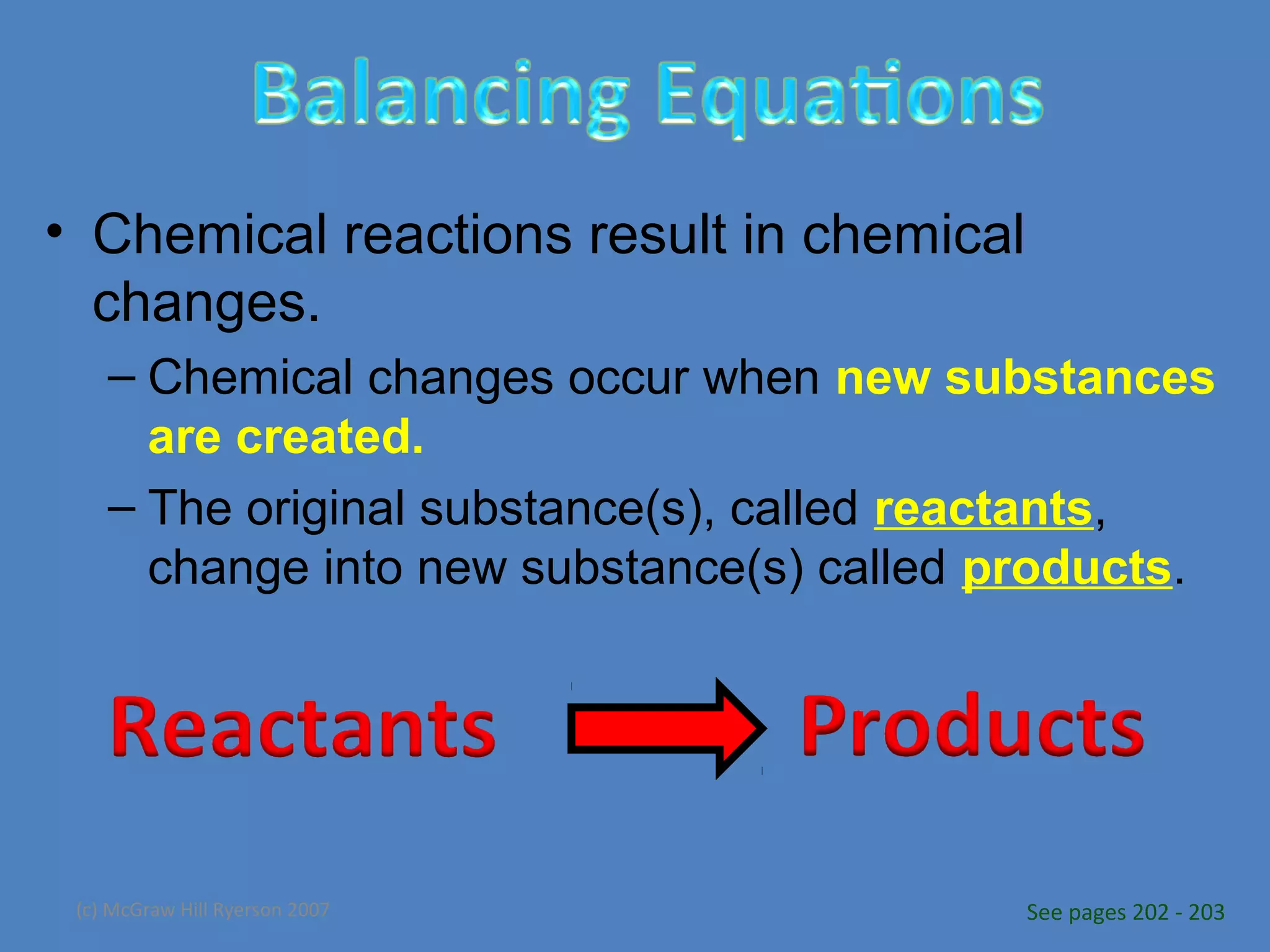
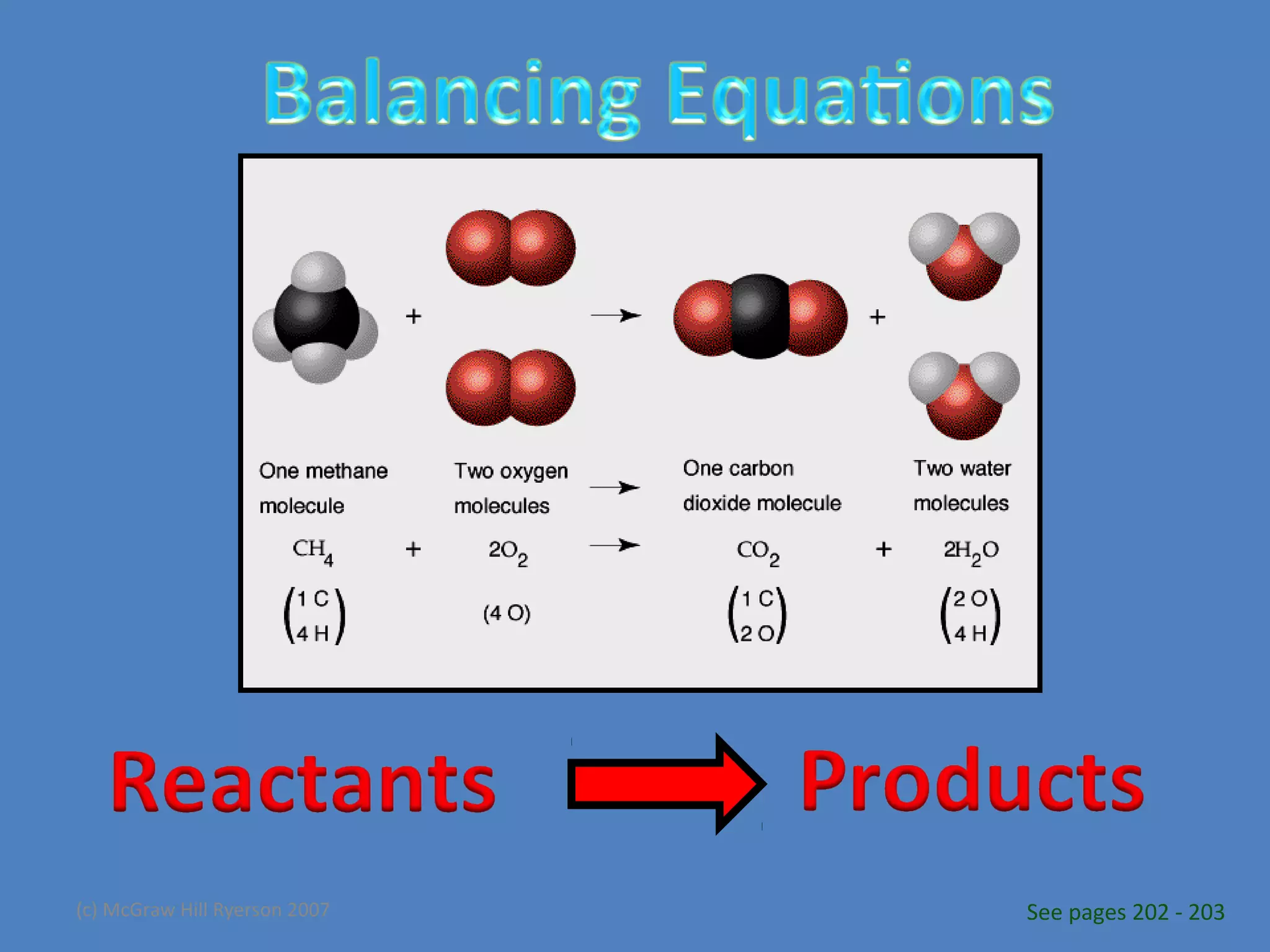
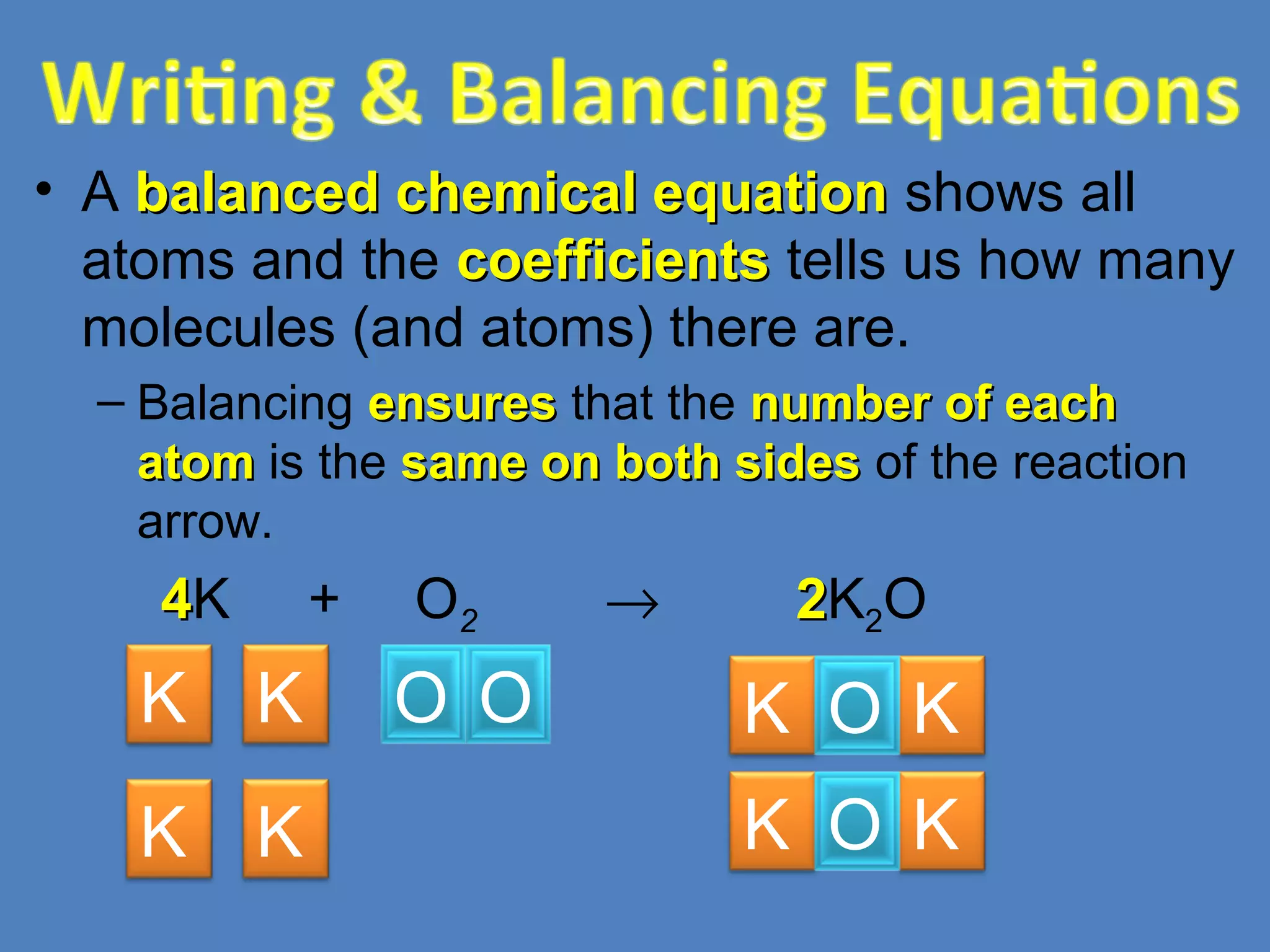
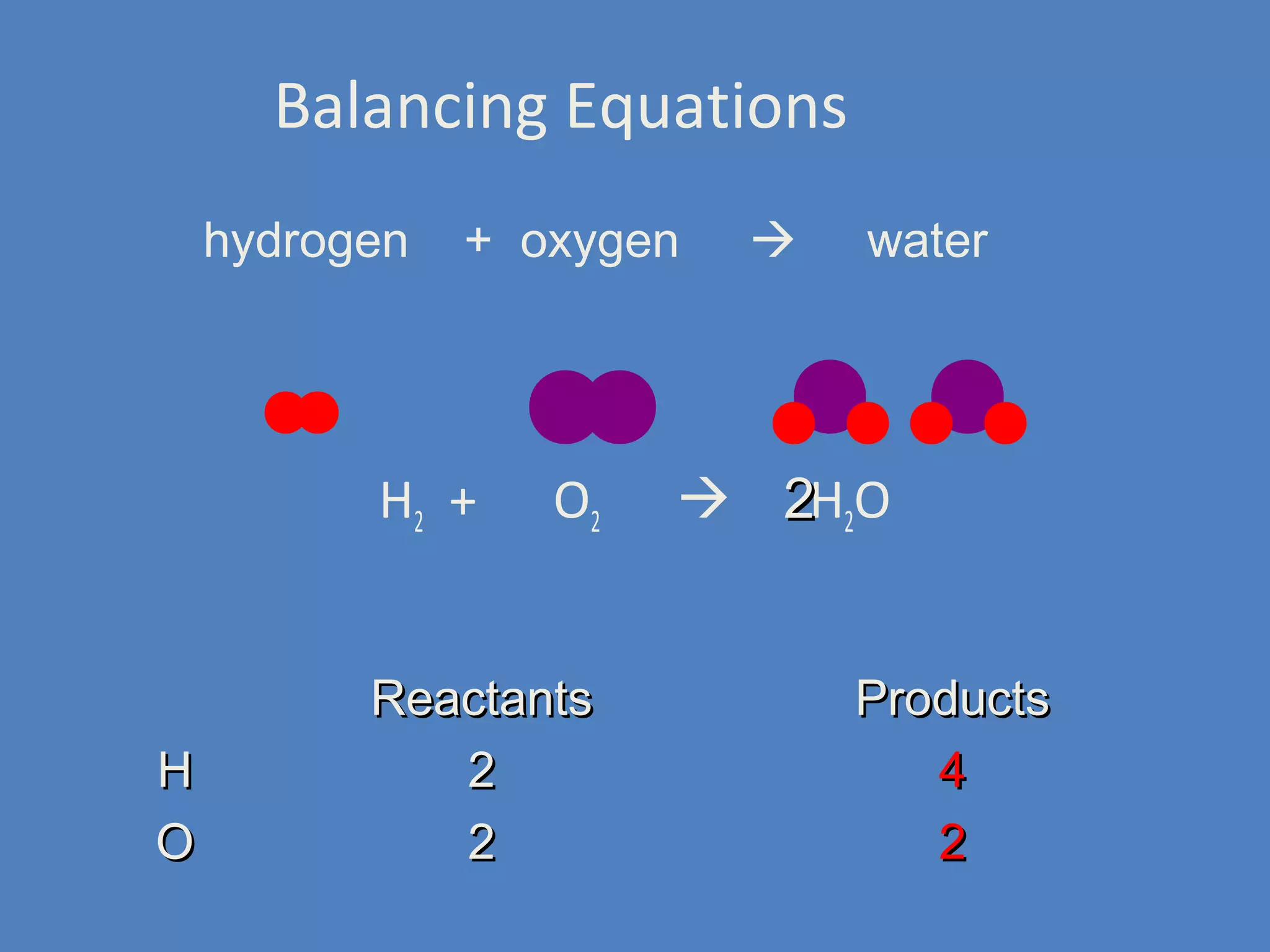
Chemical reactions result in chemical changes where reactants change into new products. Chemical reactions can be written with word equations or symbolic equations. A balanced chemical equation shows that the number of each type of atom is the same on both sides of the reaction, ensuring the law of conservation of mass is followed. Balancing chemical equations involves determining the coefficients needed for the number of atoms to be equal on both sides.