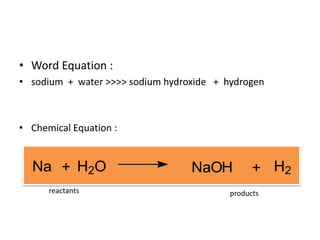
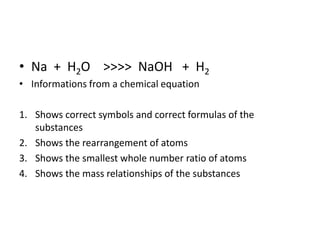
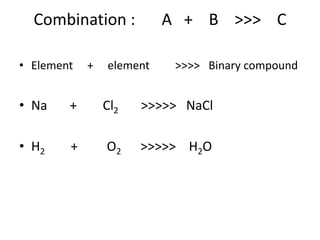
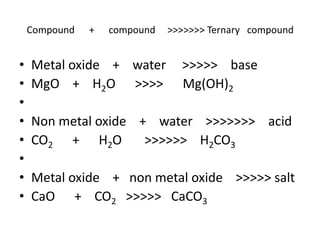
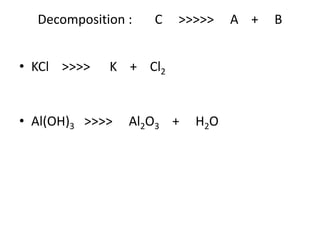
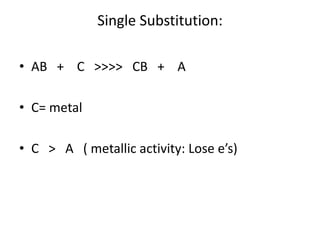
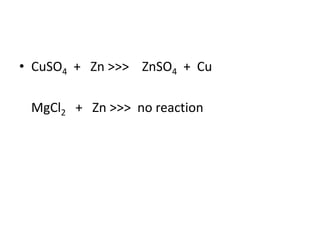
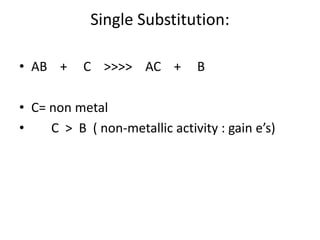
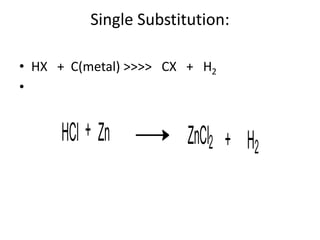
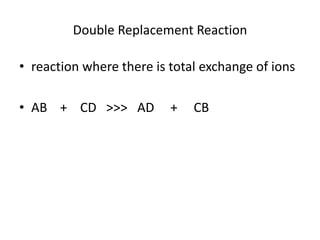
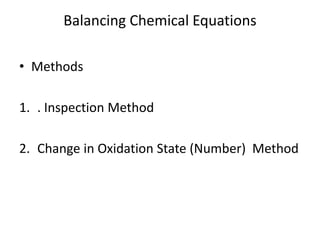
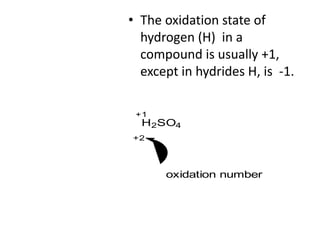
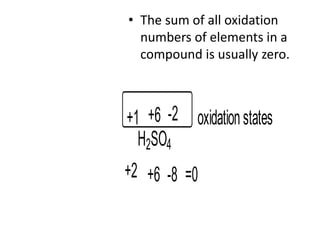
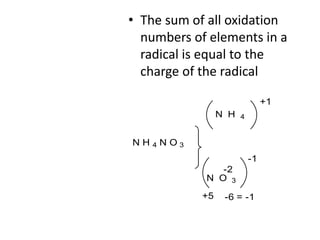
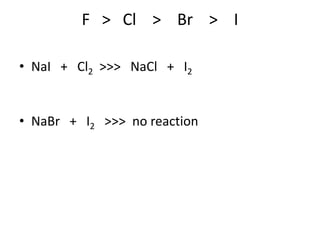Chemical equations are used to represent chemical reactions. They show the reactants on the left and products on the right, connected by an arrow. Symbols and chemical formulas are used to indicate the specific elements and compounds. Key information provided by a chemical equation includes the identities and ratios of reactants and products, and the rearrangement of atoms. Chemical equations can be classified based on the type of reaction, such as combination, decomposition, single or double displacement. They must be balanced so the number of atoms of each element is equal on both sides. This can be done through inspection or by considering changes in oxidation states.