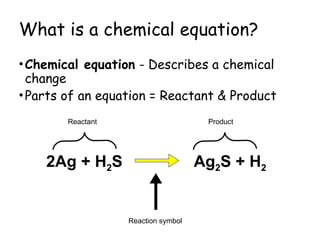
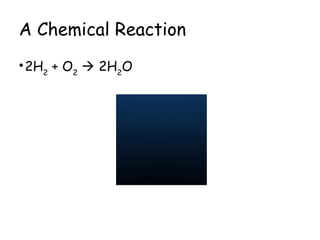
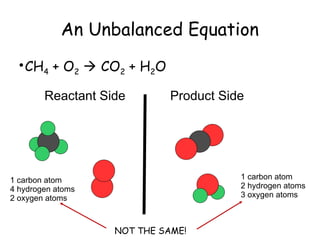
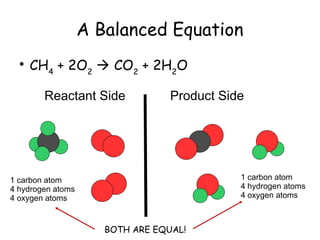
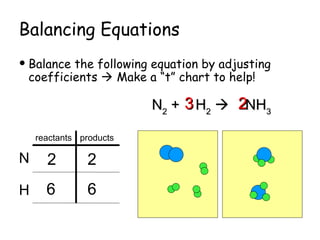
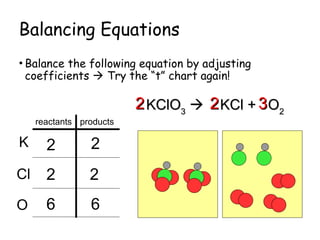
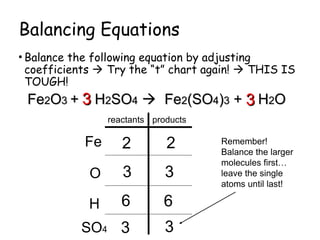
The document explains the concept of balancing chemical equations, detailing the roles of reactants and products. It emphasizes the law of conservation of mass, which states that matter is neither created nor destroyed during a chemical reaction, thereby maintaining an equal number of atoms on both sides of an equation. Additionally, it outlines rules for balancing equations, such as changing only coefficients and provides strategies and tips for effectively balancing chemical reactions.