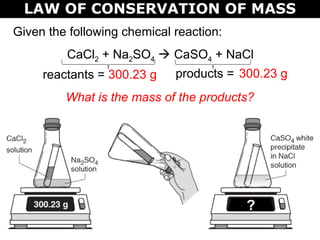
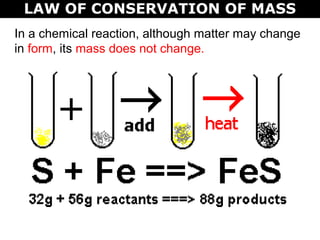
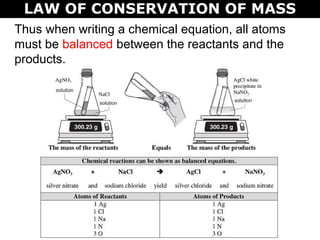
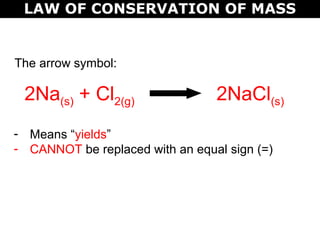
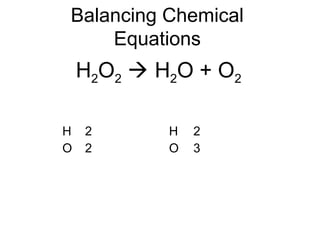
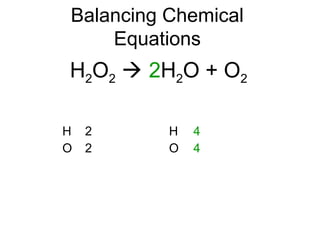
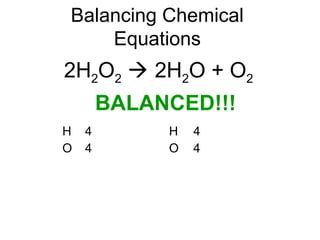
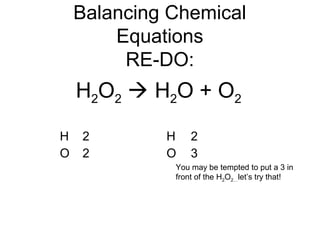
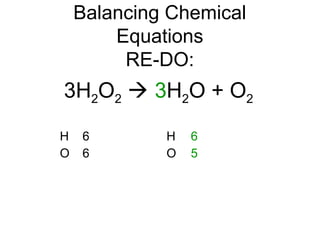
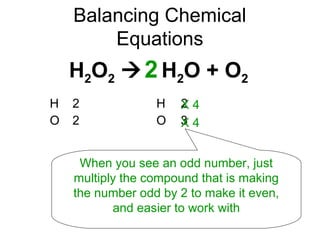
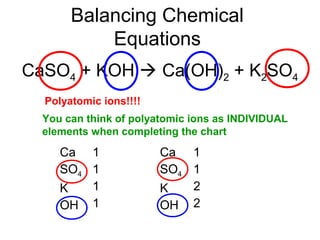
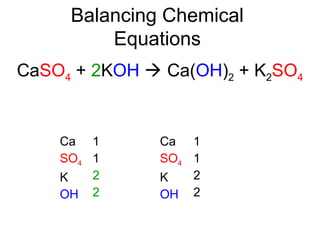
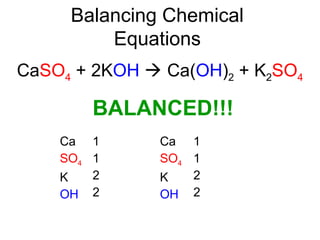
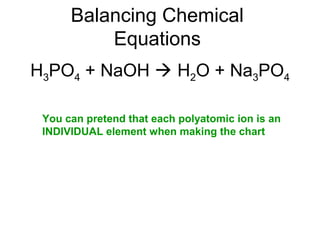
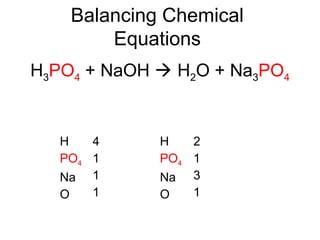
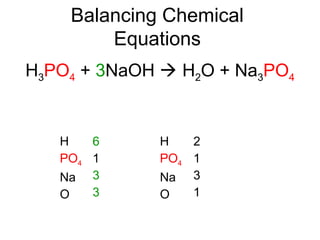
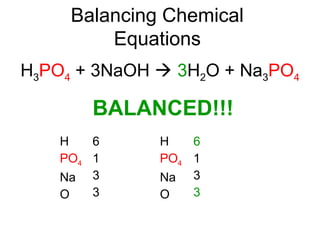
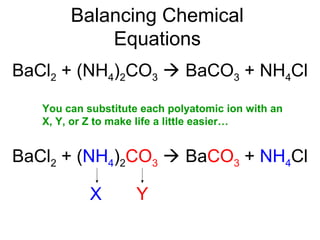
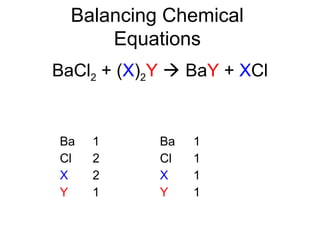
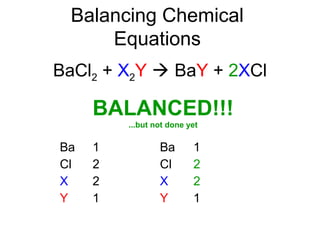
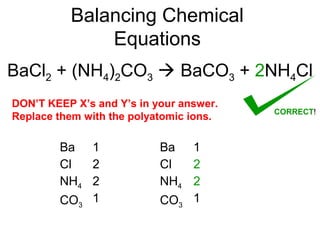
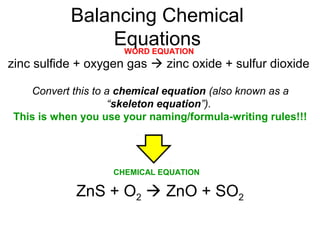
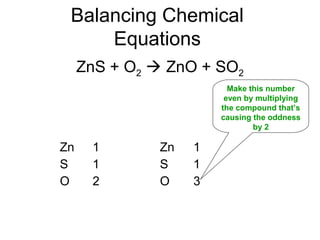
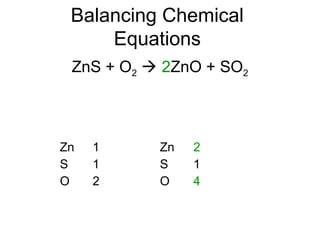
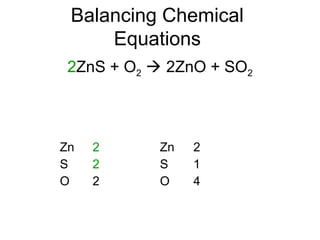
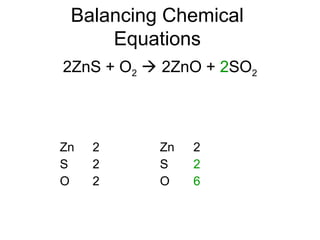
The document discusses the law of conservation of mass and balancing chemical equations. It explains that in a chemical reaction, while the types of atoms may change, the total mass remains the same. It then provides examples of balancing different chemical equations by making the numbers of atoms equal on both sides of the reaction arrow. Methods for balancing include multiplying coefficients and accounting for polyatomic ions.