The document provides an introduction to chemical reactions, including:
- Chemical reactions involve breaking bonds in reactants and forming new bonds in products.
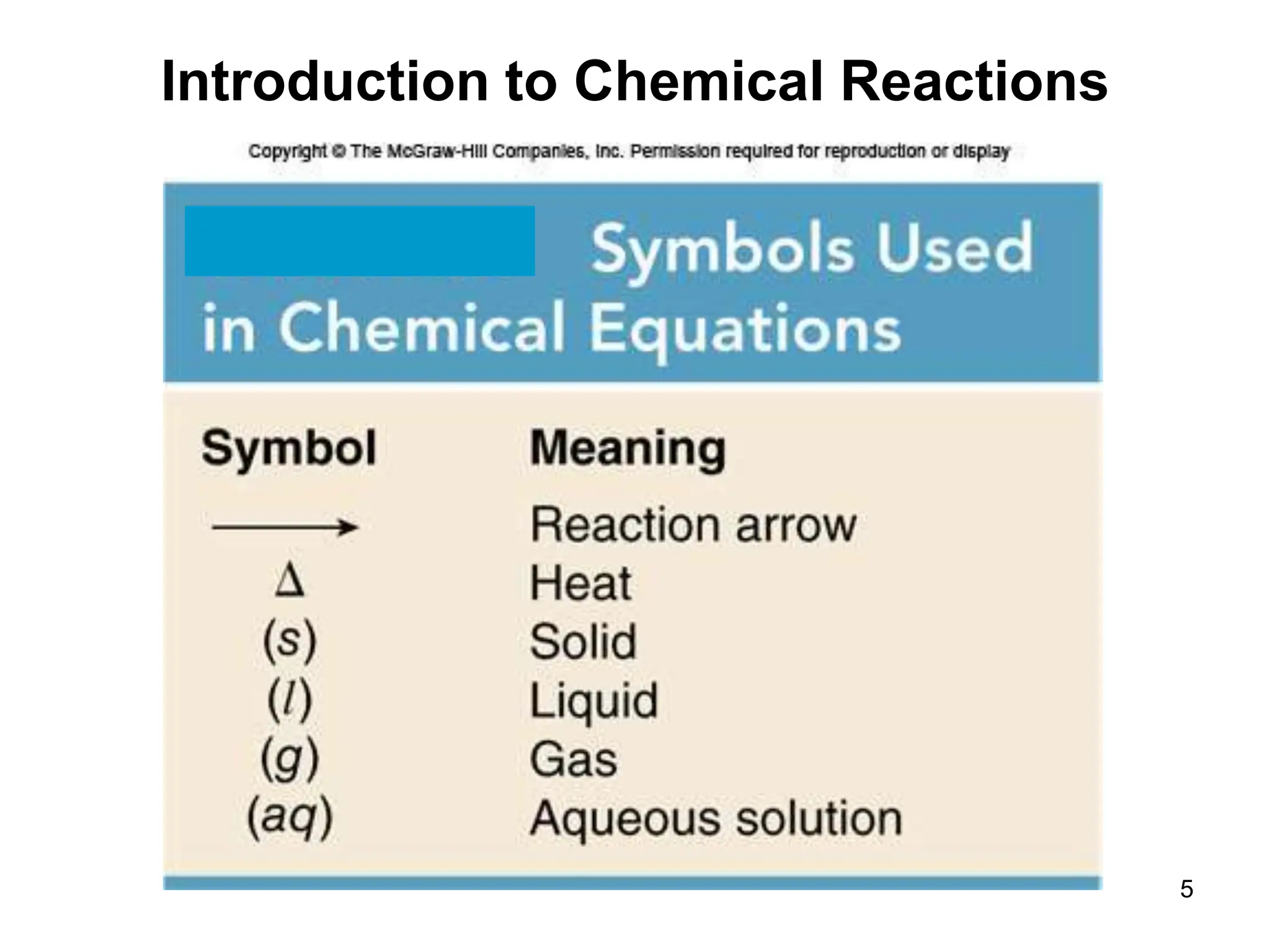
- A chemical equation uses symbols to represent reactants and products, with coefficients showing quantities.
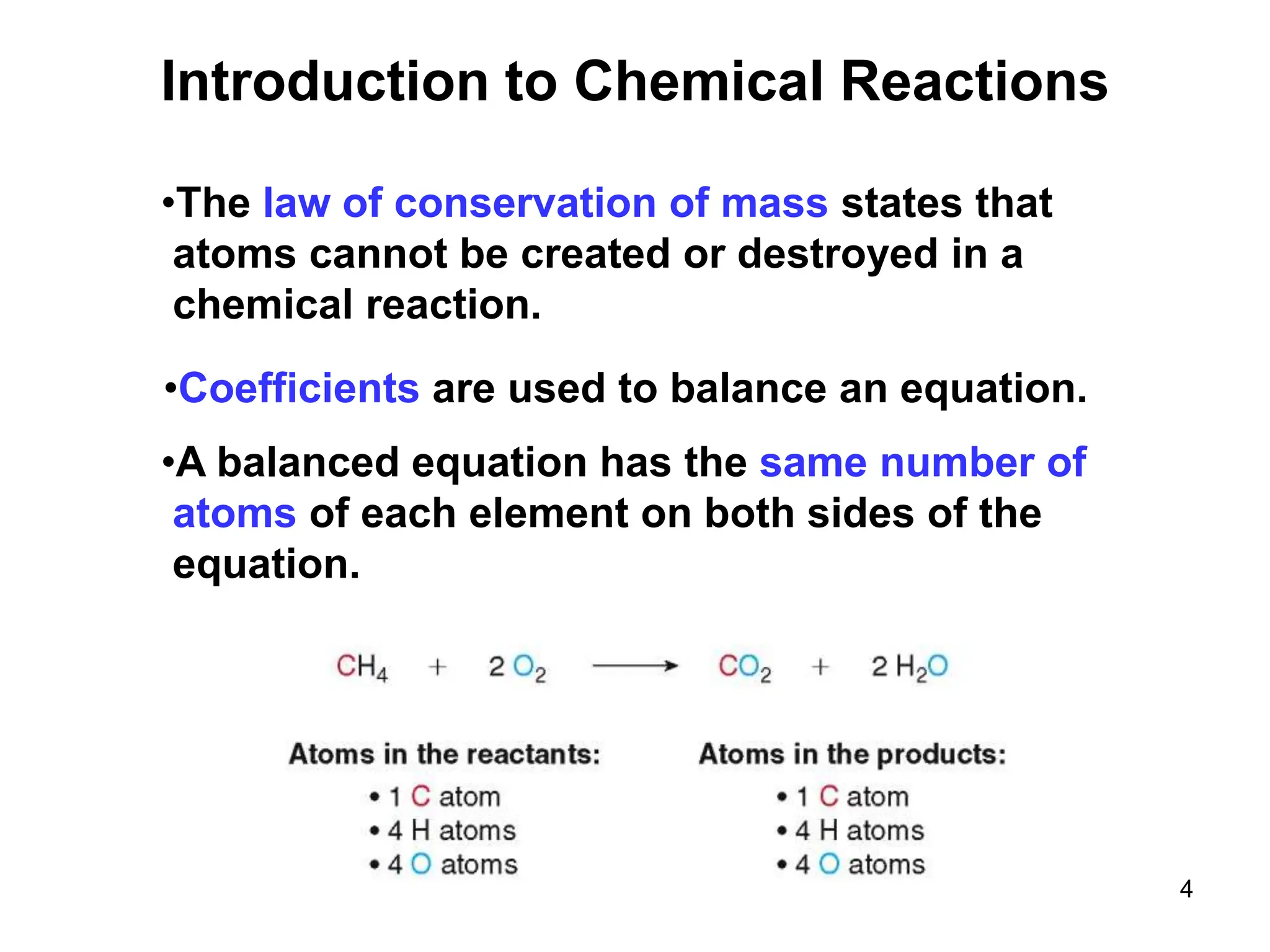
- The law of conservation of mass states atoms are not created or destroyed in a reaction.
- Evidence of a chemical reaction includes changes in color, temperature, gas evolution, or precipitation.



![7
Balancing Chemical Equations
HOW TO Balance a Chemical Equation
Example Write a balanced chemical equation for
the reaction of propane (C3H8) with
oxygen (O2) to form carbon dioxide (CO2)
and water (H2O).
Step [1] Write the equation with the correct formulas.
C3H8 + O2 CO2 + H2O
•The subscripts in a formula can never be changed
to balance an equation, because changing a
subscript changes the identity of a compound.](https://image.slidesharecdn.com/460370723-1-chemical-reactions-ppt-240408213110-cdbfd141/75/46037073323-1-Chemical-Reactions-ppt-ppt-7-2048.jpg)
![8
Balancing Chemical Equations
Step [2]
HOW TO Balance a Chemical Equation
Balance the equation with coefficients one
element at a time.
•Balance the C’s first:
•Balance the H’s next:](https://image.slidesharecdn.com/460370723-1-chemical-reactions-ppt-240408213110-cdbfd141/75/46037073323-1-Chemical-Reactions-ppt-ppt-8-2048.jpg)
![9
Balancing Chemical Equations
Step [2]
HOW TO Balance a Chemical Equation
Balance the equation with coefficients one
element at a time.
•Finally, balance the O’s:](https://image.slidesharecdn.com/460370723-1-chemical-reactions-ppt-240408213110-cdbfd141/75/46037073323-1-Chemical-Reactions-ppt-ppt-9-2048.jpg)
![10
Balancing Chemical Equations
Step [3] Check to make sure that the smallest set
of whole numbers is used.
HOW TO Balance a Chemical Equation](https://image.slidesharecdn.com/460370723-1-chemical-reactions-ppt-240408213110-cdbfd141/75/46037073323-1-Chemical-Reactions-ppt-ppt-10-2048.jpg)







