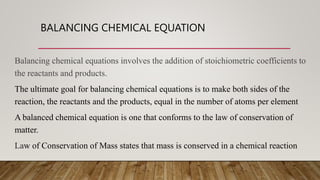
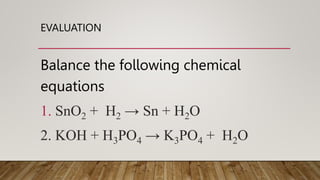
1. The document discusses balancing chemical equations, which involves adding coefficients to reactants and products to make both sides equal in terms of atoms. This ensures mass is conserved, as the total mass of reactants must equal the total mass of products.
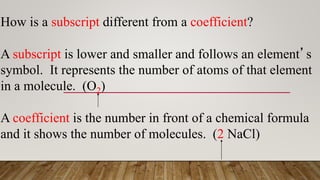
2. Rules for balancing equations include writing unbalanced formulas with an arrow between reactants and products, counting atoms on each side, and balancing one element at a time using the lowest common multiple as the coefficient.
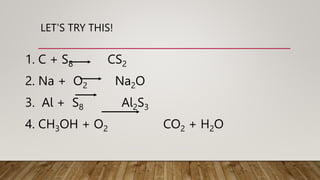
3. Examples show balancing equations by adding coefficients like 2 or 3 in front of formulas to make atom counts equal on both sides.