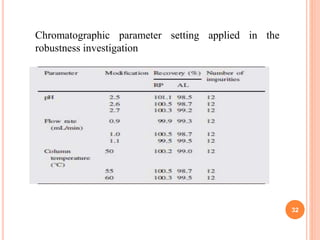
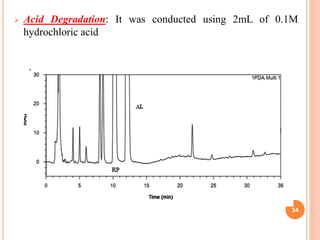
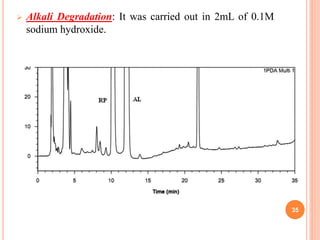
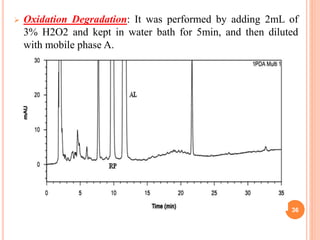
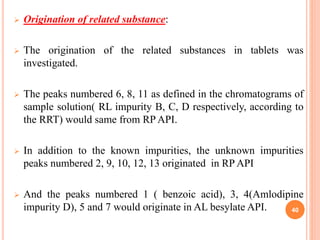
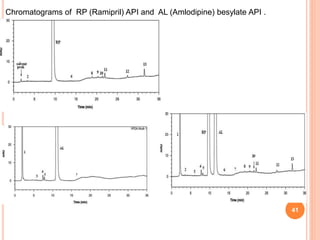
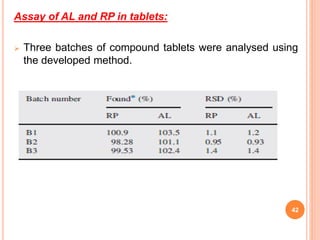
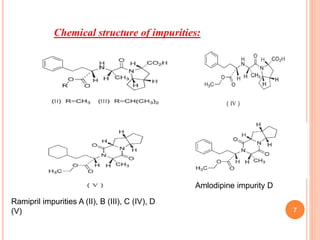
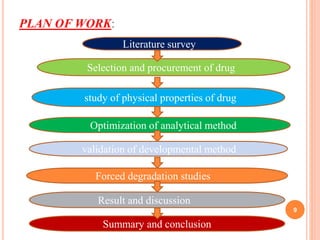
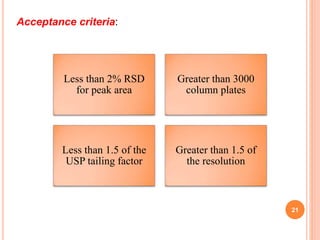
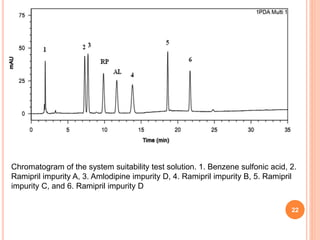
This document describes the development and validation of an RP-HPLC method for the simultaneous determination of ramipril and amlodipine in tablets. The method utilizes a gradient elution with a C18 column, mobile phase of acetonitrile and sodium perchlorate buffer, and UV detection. The method was validated per ICH guidelines and showed good linearity, accuracy, precision, specificity and robustness. Forced degradation studies demonstrated the method can separate ramipril, amlodipine and their degradation products. The method was successfully applied to determine the content of ramipril and amlodipine in three tablet batches.





![DRUG PROFILE
6
RAMIPRIL AMLODIPINE
STUCTURE
CHEMICAL
NAME
[(2S, 3aS, 6aS)-1-[(S)-2-
[[(S)-1-
(ethoxycarbonyl)-3-
phenylpropyl] amino]
propanoyl] octahydro
cyclopenta [b] pyrrole-2-
carboxylic acid
[3-Ethyl5-methyl(4RS)-2-[(2-
aminoethoxy)
methyl]-4-(2-chlorophenyl)-
6-methyl-1,4-
dihydropyridine-3,5-
dicarboxylate
SIDE
EFFECTS
Hypotension, cough Peripheral edema](https://image.slidesharecdn.com/rahulppt-140731214224-phpapp01/85/DEVELOPMENT-AND-VALIDATION-OF-AN-RP-HPLC-METHOD-FOR-SIMULTANEOUS-DETERMINATION-OF-RAMIPRIL-AND-AMLODIPINE-IN-TABLETS-6-320.jpg)














![27
Accuracy results [Recovery (%)] for the determination of AL
(Amlodipine) and RP (Ramipril).](https://image.slidesharecdn.com/rahulppt-140731214224-phpapp01/85/DEVELOPMENT-AND-VALIDATION-OF-AN-RP-HPLC-METHOD-FOR-SIMULTANEOUS-DETERMINATION-OF-RAMIPRIL-AND-AMLODIPINE-IN-TABLETS-27-320.jpg)