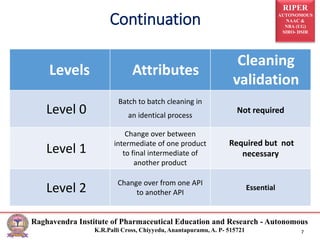
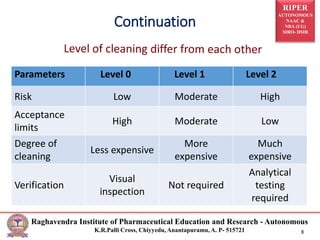
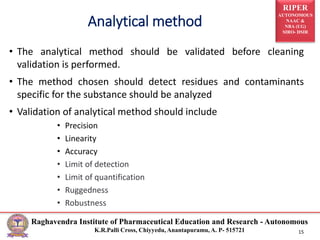
The document discusses the development of cleaning methods in pharmaceutical education, emphasizing stages such as feasibility, development, and validation. It outlines various cleaning agents, sampling techniques, and parameters for cleaning validation levels, detailing acceptance limits and the analytical methods required for ensuring effectiveness. Additionally, it addresses the importance of selection and validation of cleaning processes to maintain product safety and compliance.