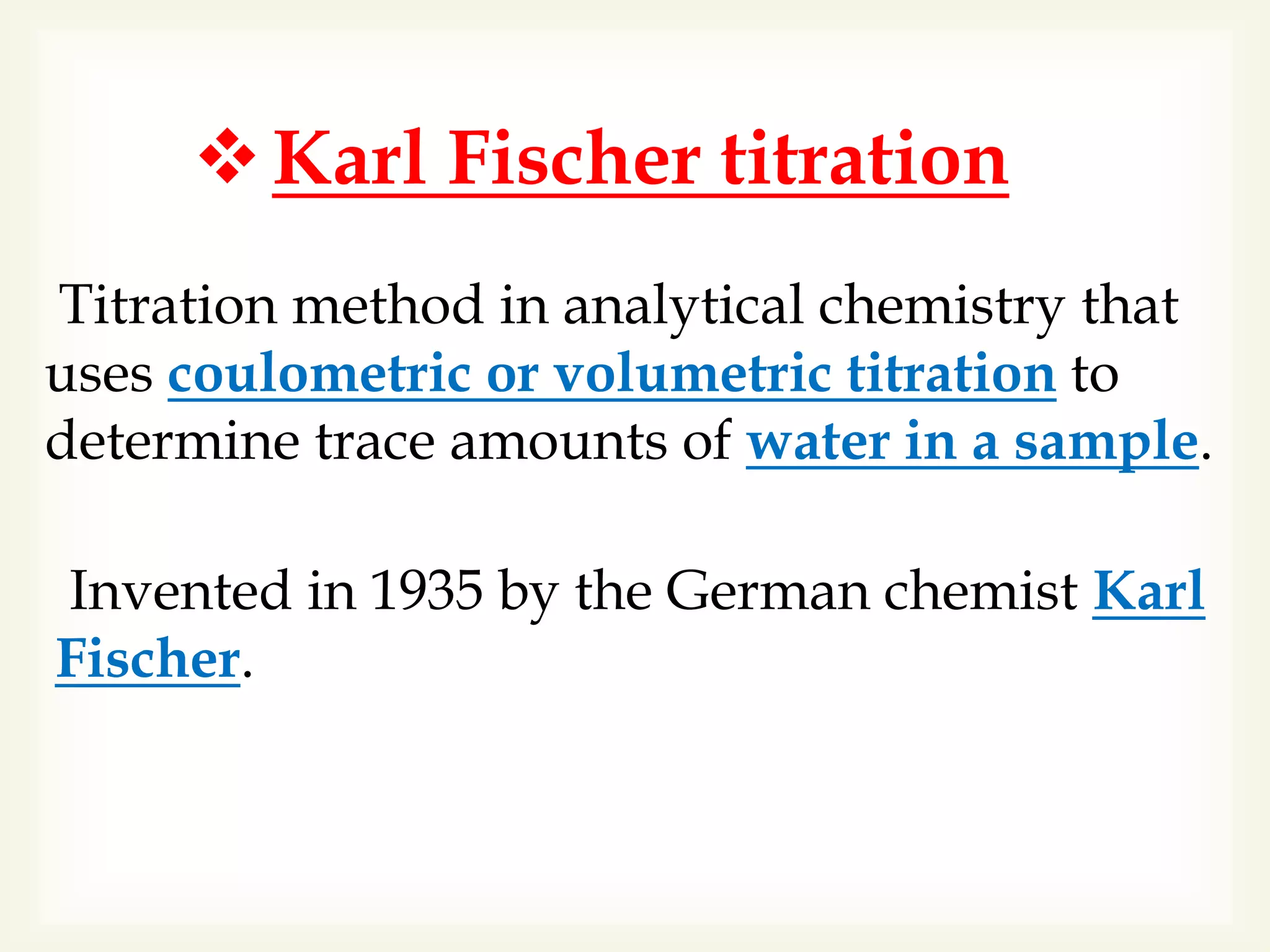
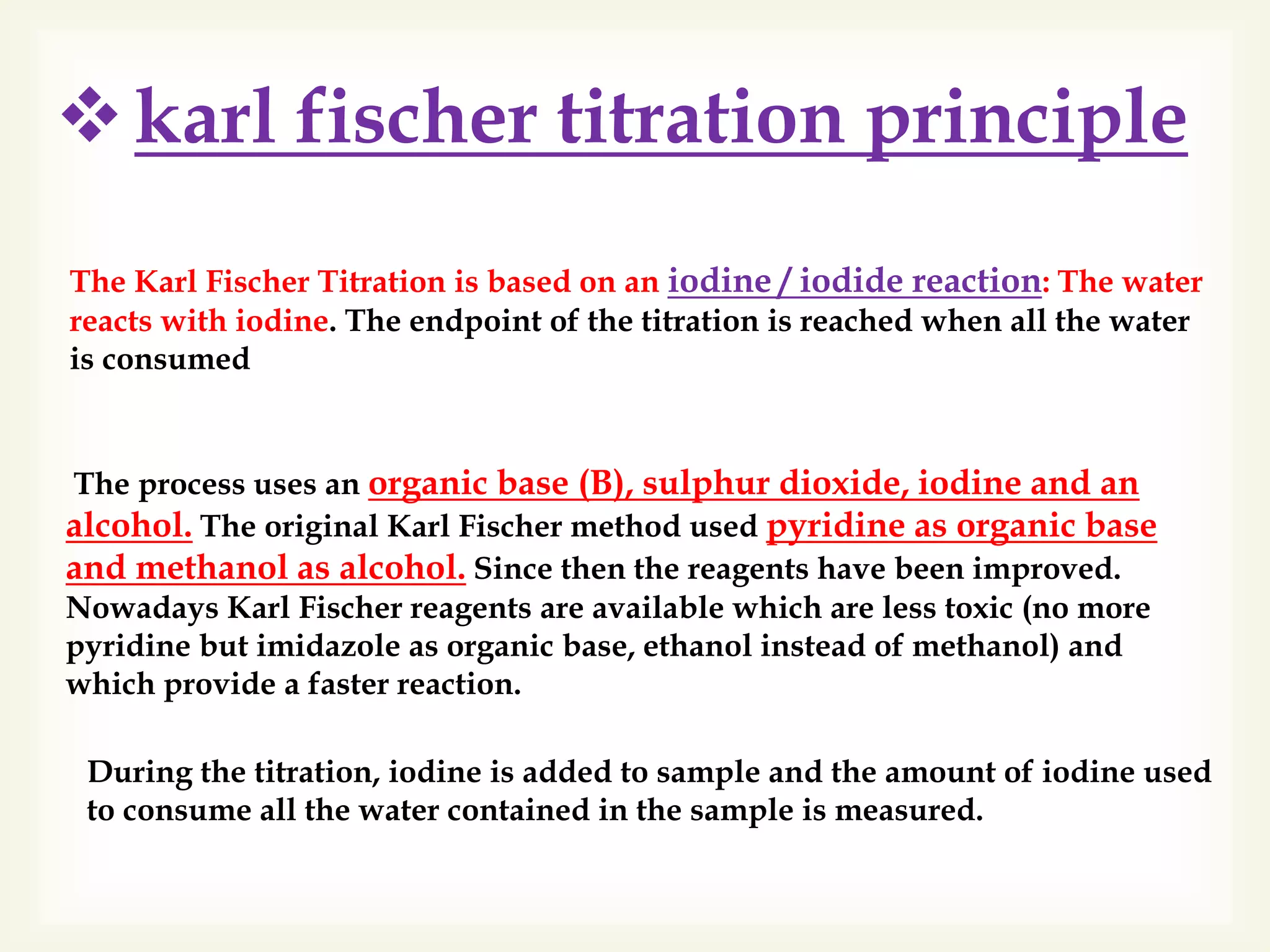
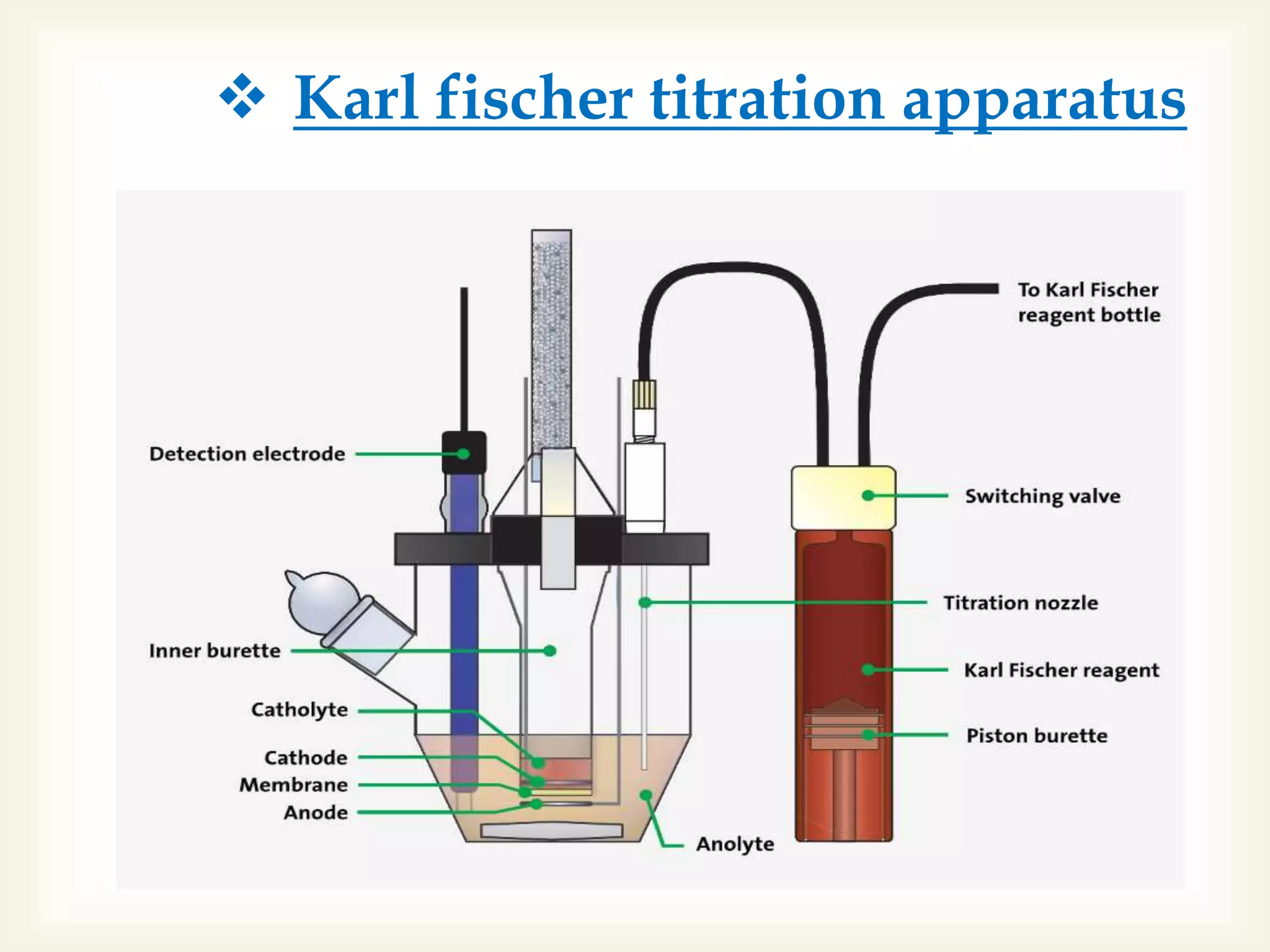
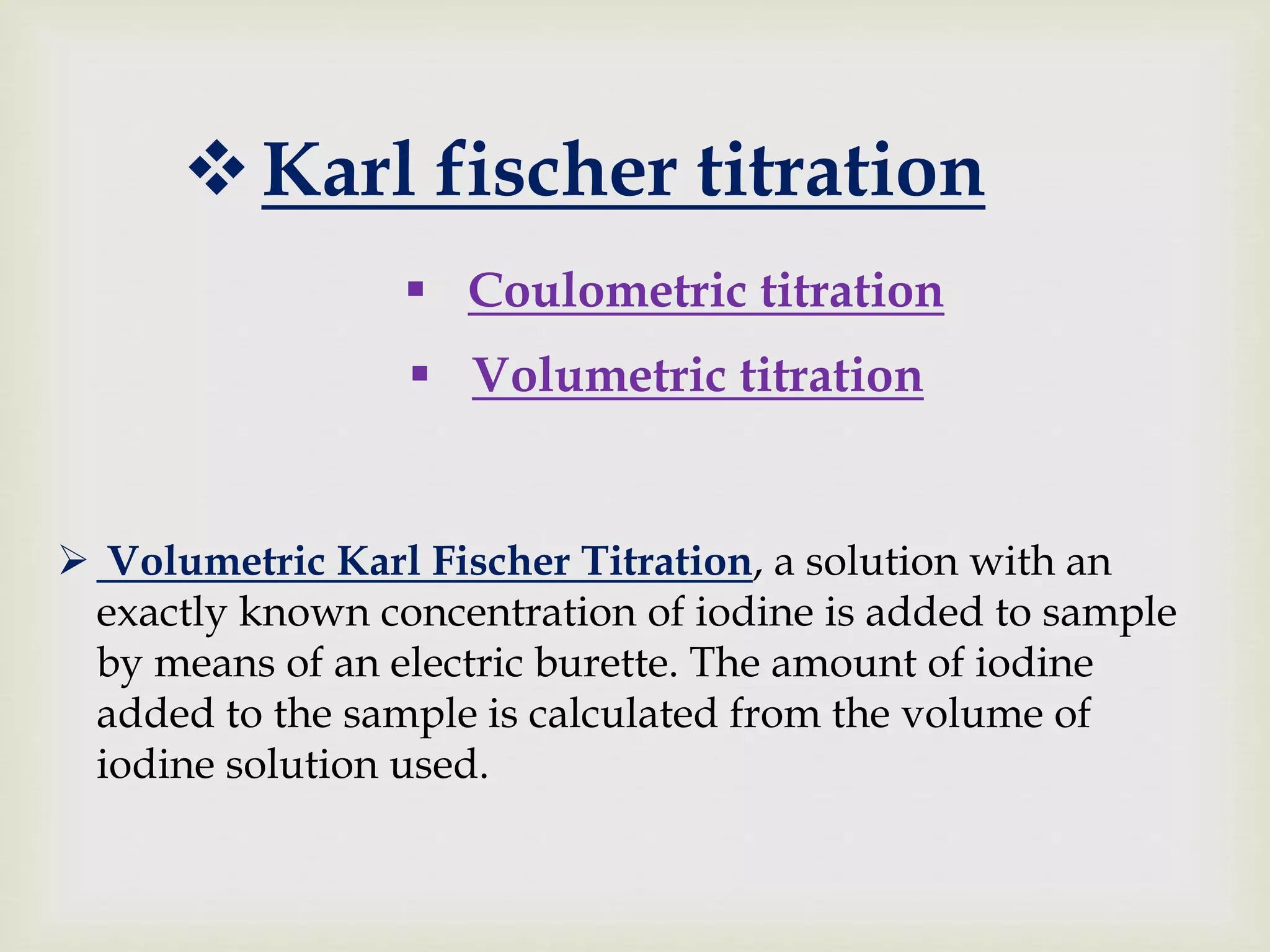
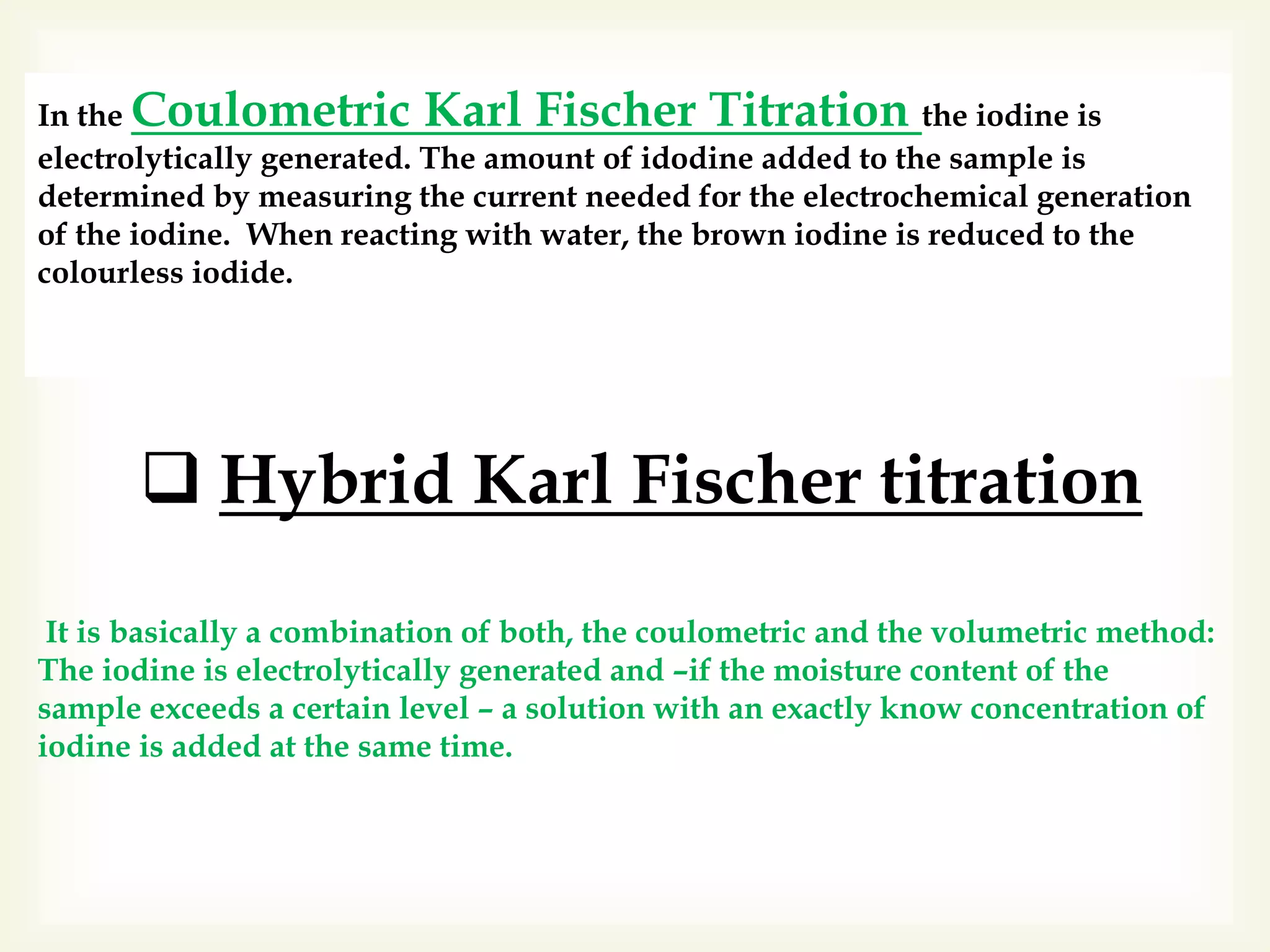
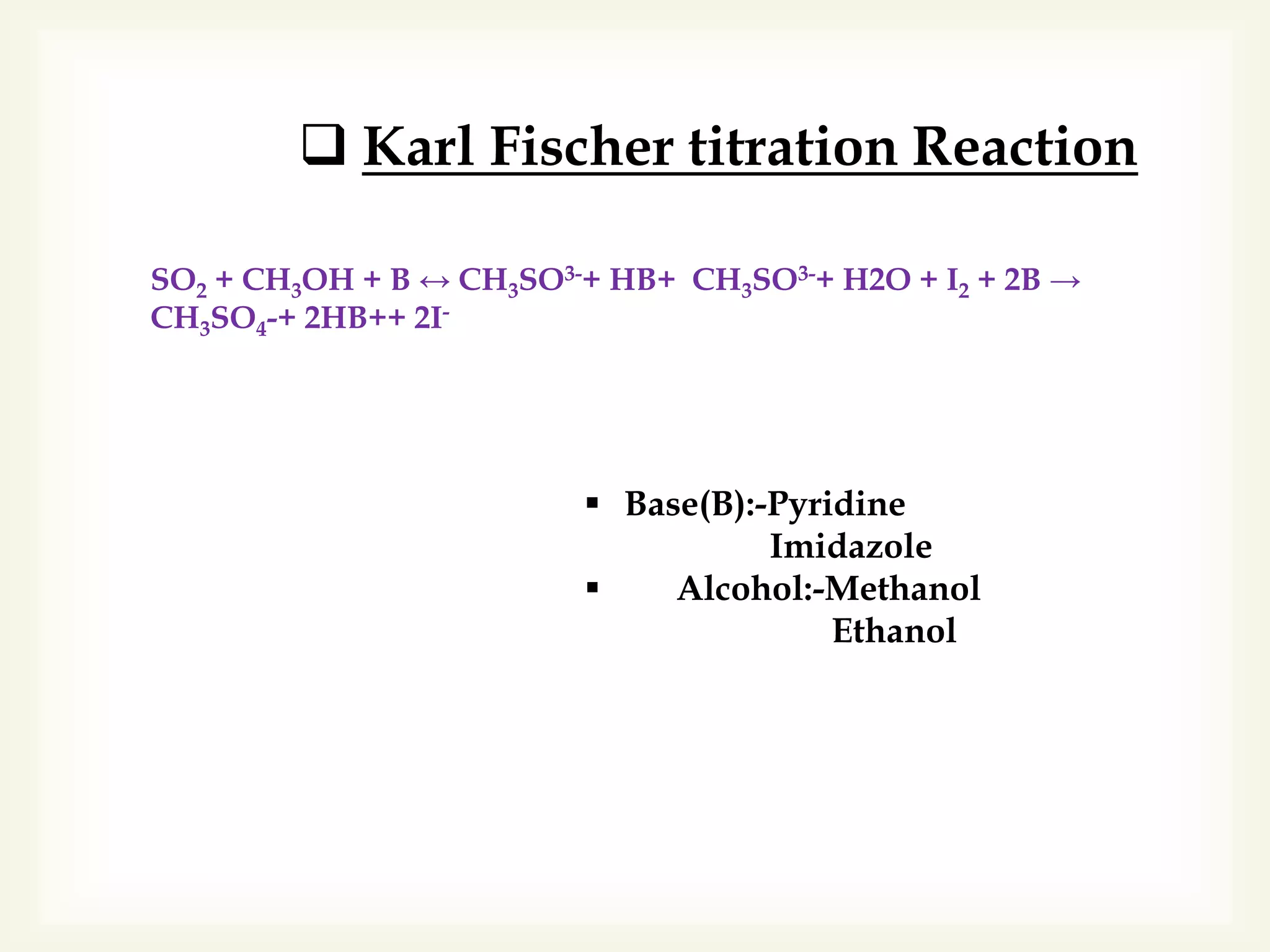
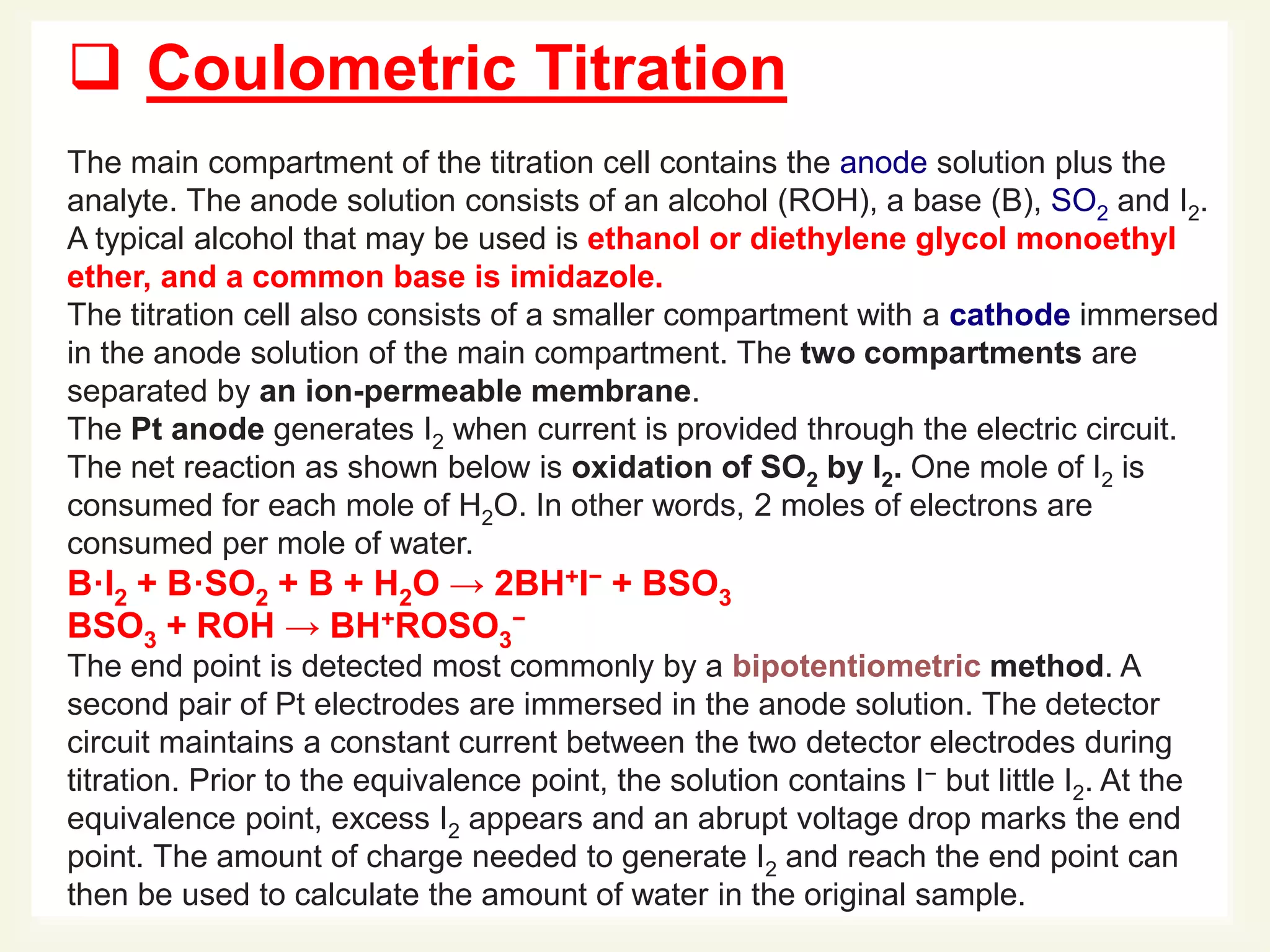
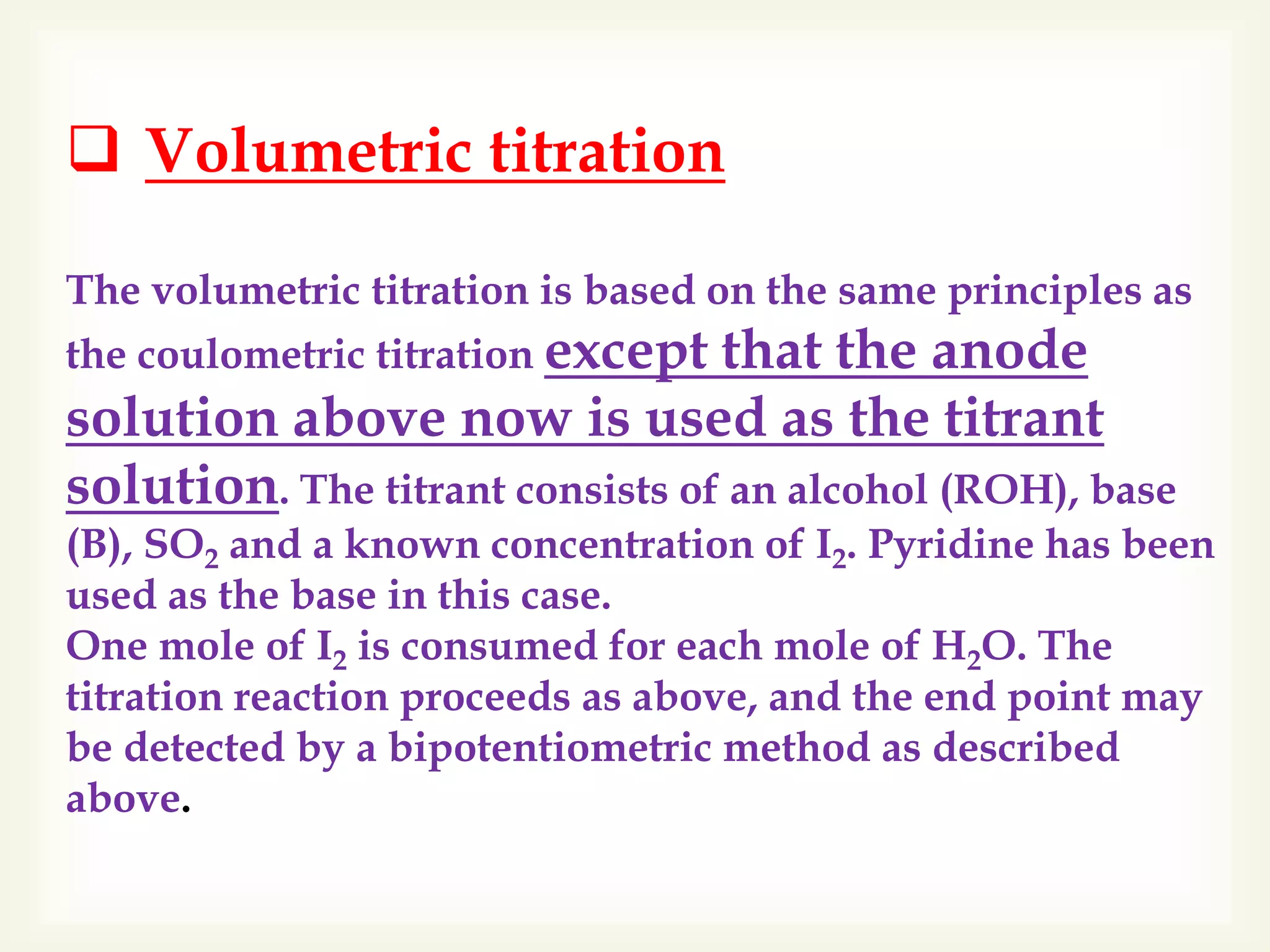
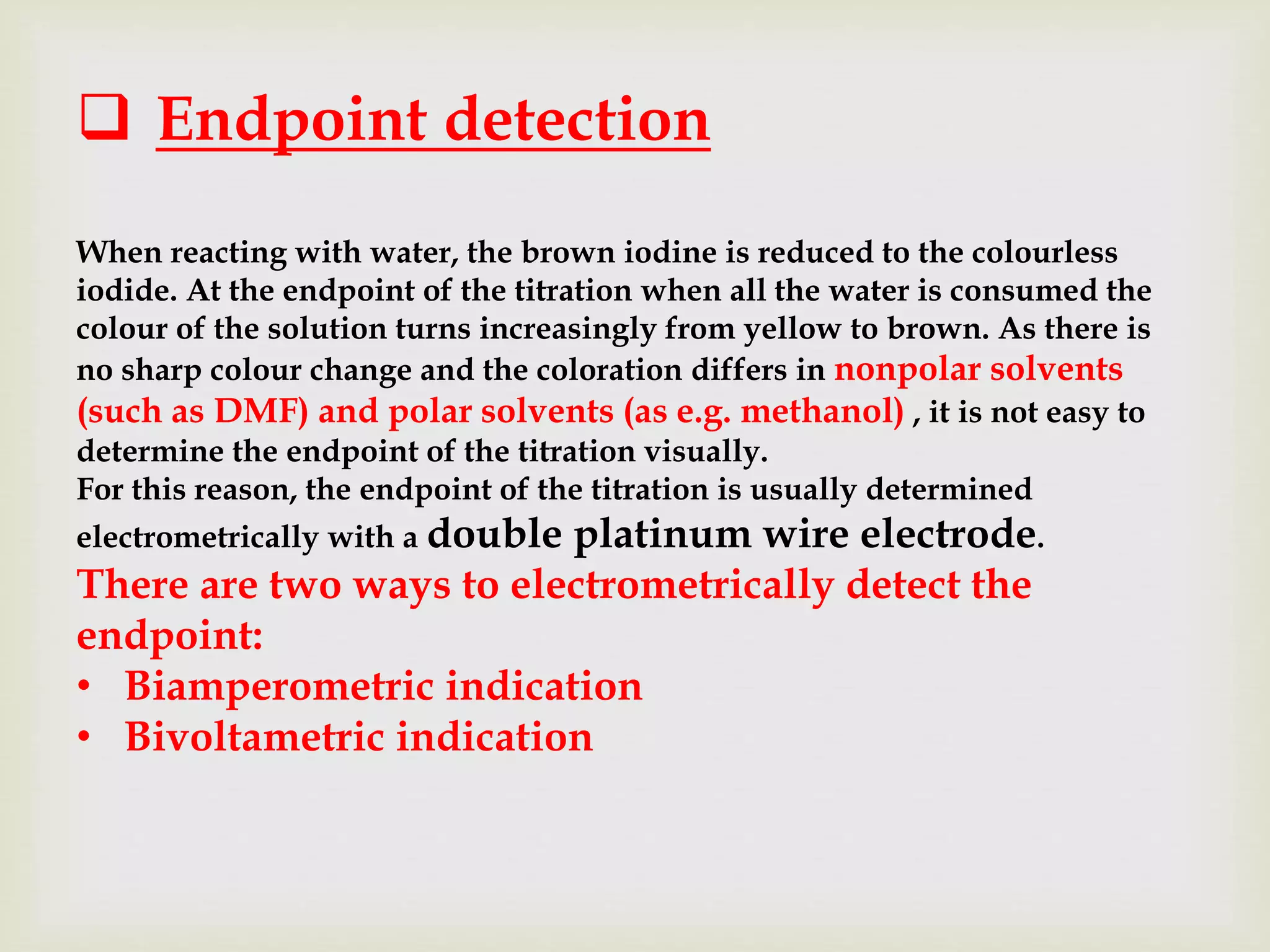
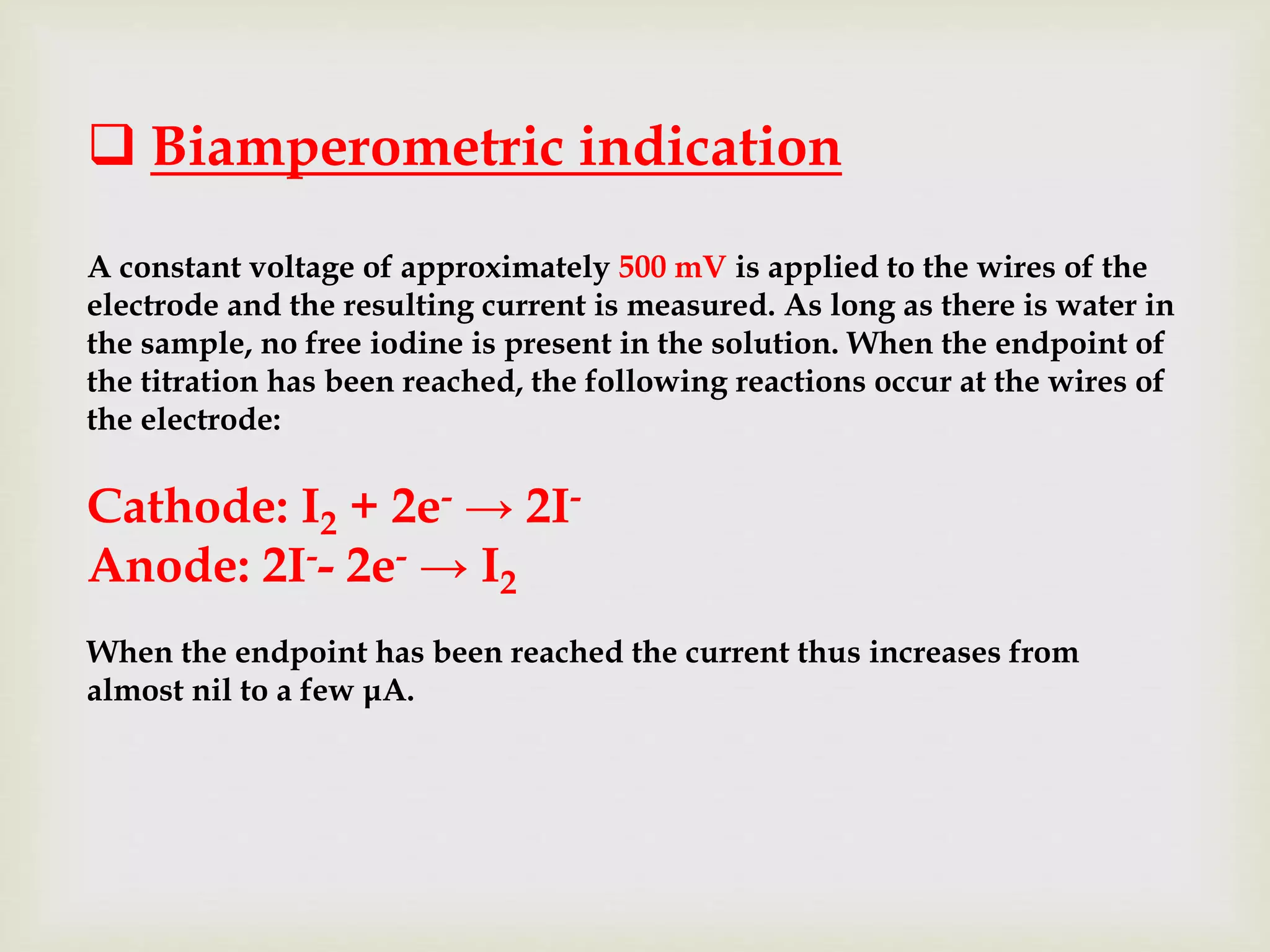
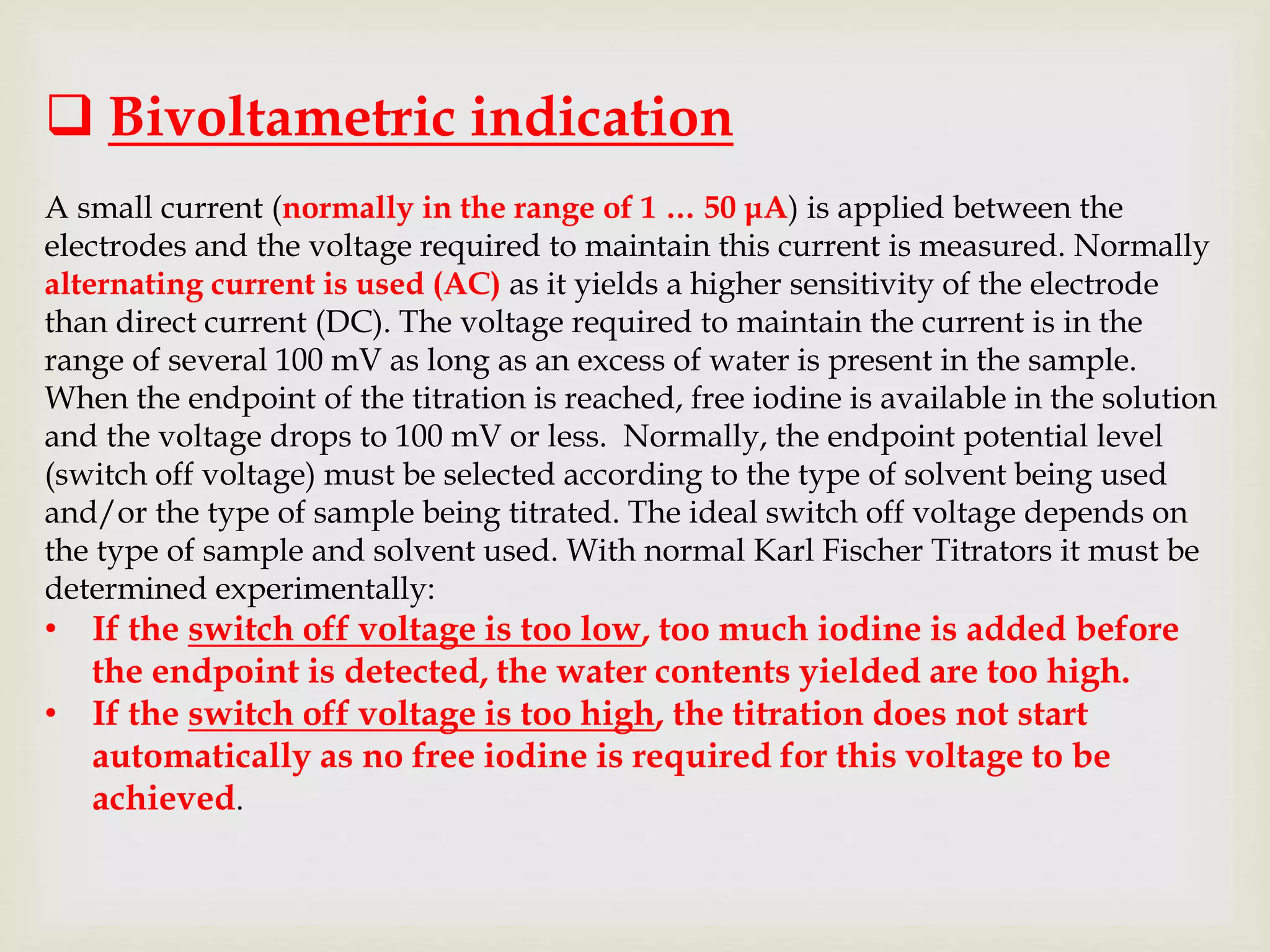
The Karl Fischer titration is an analytical chemistry method used to determine trace amounts of water in a sample, developed by German chemist Karl Fischer in 1935. It involves a reaction between iodine and water, with various reagents used over time to enhance safety and reaction speed, including different organic bases like imidazole and alcohols like ethanol. The titration can be performed volumetrically or coulometrically, with specific methods suited for different water content levels in samples.

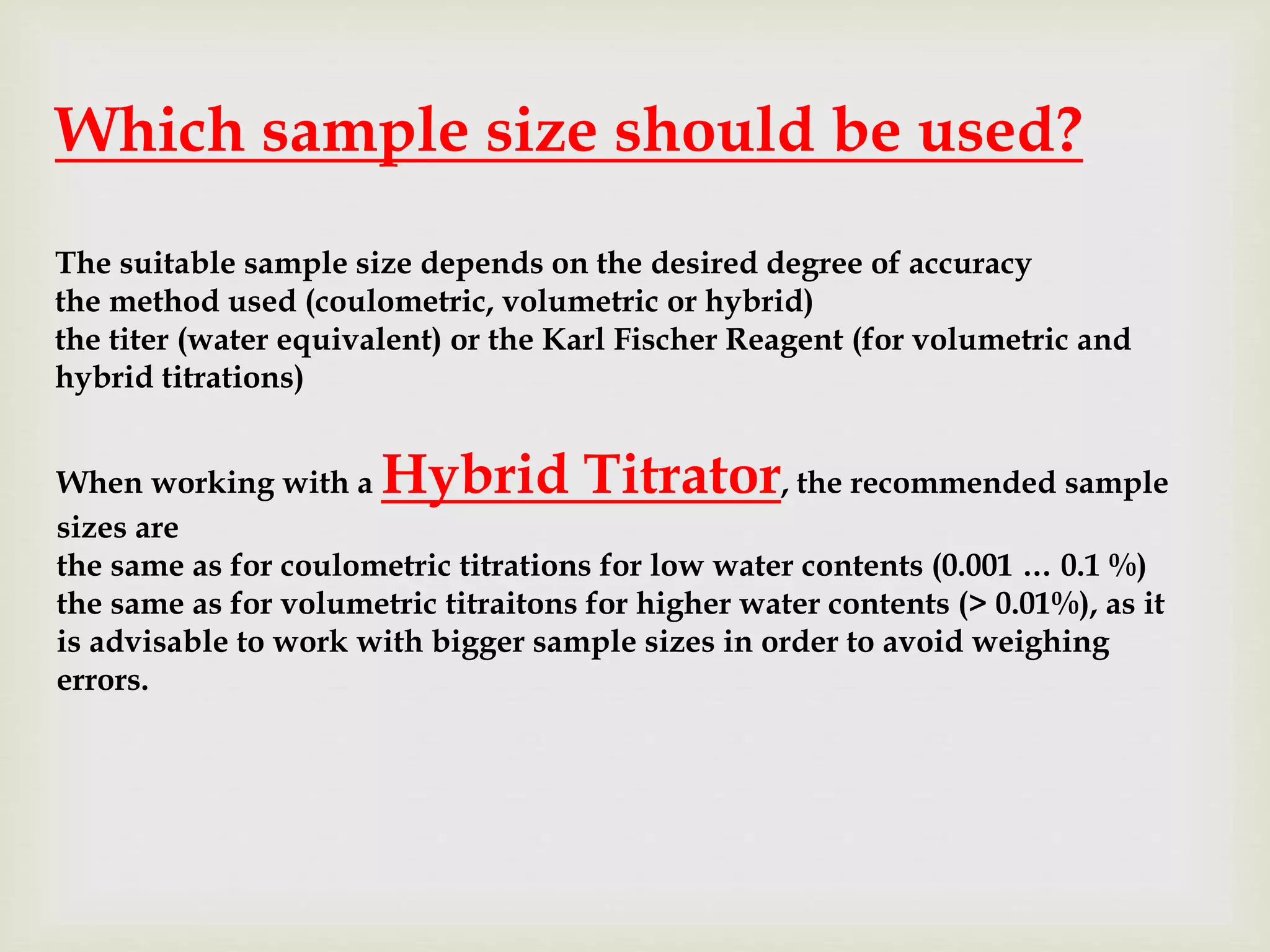
![Water content Volumetric Coulometric
[%] [ppm] WE = 2 WE = 5
0.001 10 > 25 g not recommen
ded
5 … 10 g
0.01 100 > 20 g not recommen
ded
1 … 5 g
0.1 1000 2 … 9 g 5 … 22.5 g 100 mg … 1 g
1 10000 0.2 … 0.9 g 0.5 … 2.25 g 10 mg … 100 mg
5 50000 40 … 180 mg 100 … 450 mg < 50 mg
10 100000 20 … 90 mg 50 … 225 mg < 50 mg
50 500000 not recommen
ded
< 50 mg not recommended
The table below gives a rough indication for the sample size to be used
in volumetric and coulometric titrations based on its estimated water
content.](https://image.slidesharecdn.com/4884848484848-180621062736/75/Karl-Fischer-titration-principle-apparatus-titration-types-Endpoint-detection-14-2048.jpg)