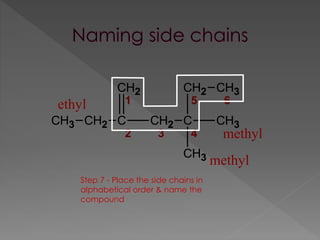
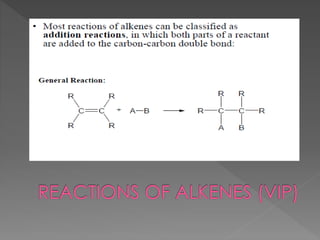
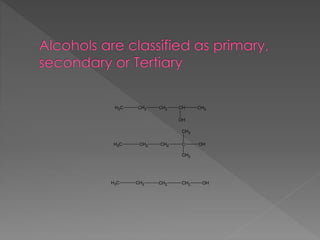
The document discusses different types of hydrocarbons including alkanes, alkenes, alkynes and cycloalkanes. It provides their general formulas and describes them as saturated or unsaturated. The document also discusses hydrocarbon naming conventions including identifying the functional group, number of carbons, side chains and their positions. Homologous series and structural isomers are introduced. Methods for producing alkenes from alkanes like dehydration of alcohols and dehydrohalogenation of haloalkanes are outlined.