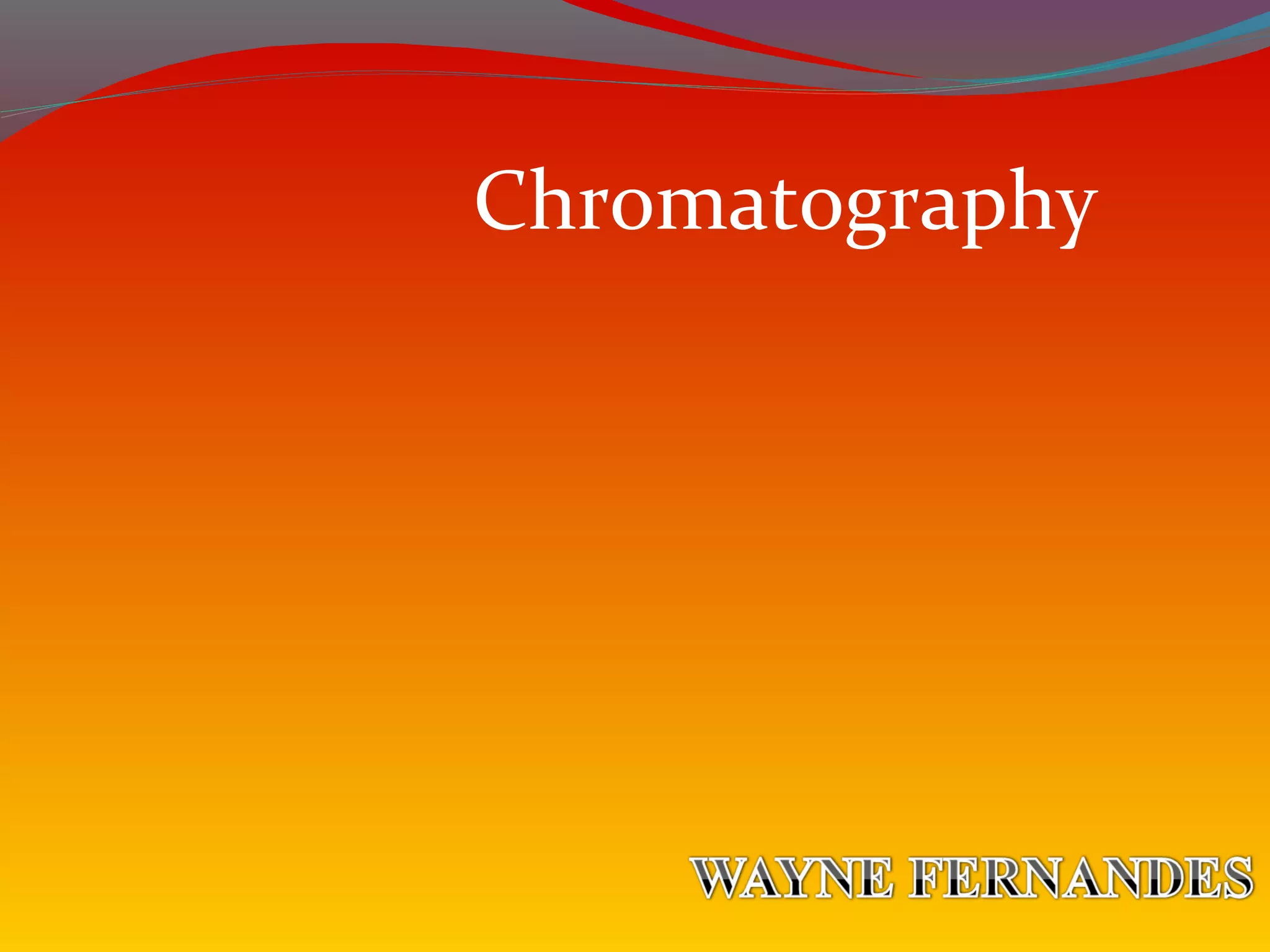
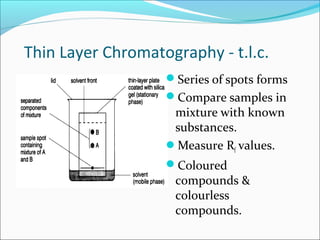
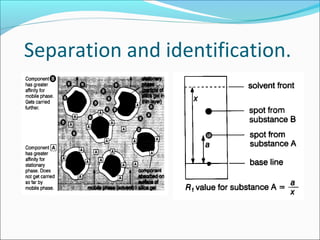
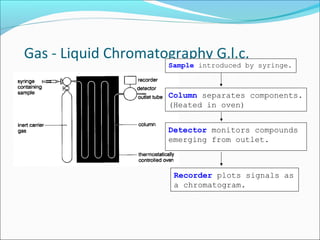
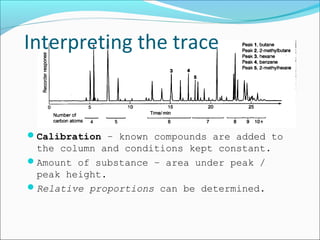
Chromatography uses the principle of partition to separate mixtures into their components based on differences in how the components interact with stationary and mobile phases. There are several types of chromatography including paper, thin layer, and gas-liquid chromatography. Gas-liquid chromatography works by introducing a sample into a column where the components are separated as they emerge based on their affinity for the mobile gas phase, with each component appearing as a separate peak on a chromatogram recording retention time. Factors like column length, packing material, carrier gas type and flow rate, and temperature can affect retention times and allow for identification of unknown components.