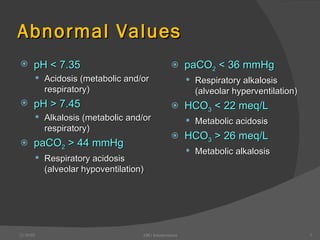
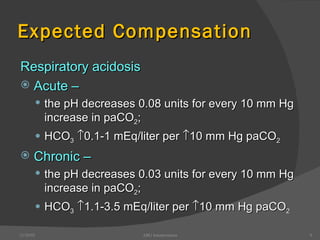
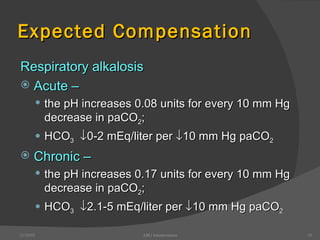
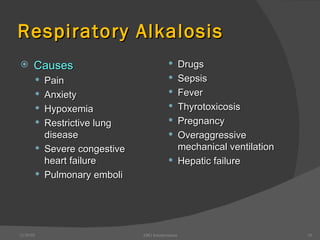
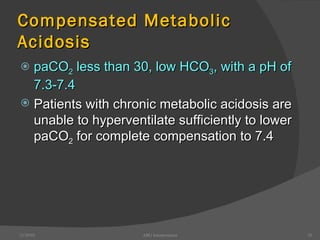
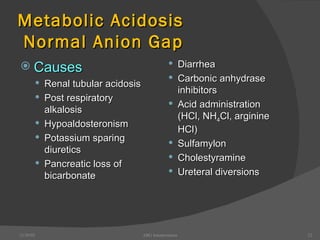
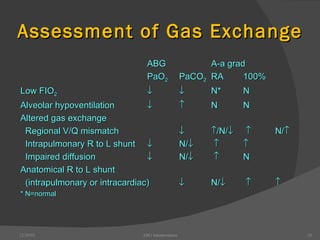
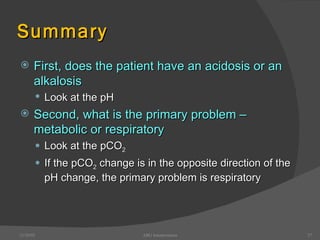
This document provides guidance on interpreting arterial blood gas (ABG) results. It discusses evaluating ABG values to determine if a patient has acidosis or alkalosis, and whether the primary problem is respiratory or metabolic. It also covers how to assess for compensatory responses and the effectiveness of oxygenation. Normal and abnormal ABG value ranges are defined. Various acid-base imbalances are described along with their typical causes and presentations. Case examples are also included to demonstrate applying the ABG interpretation process.