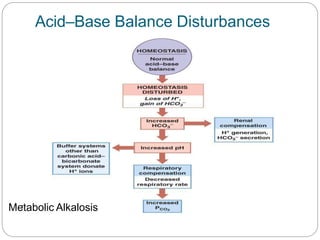
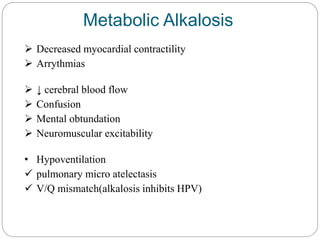
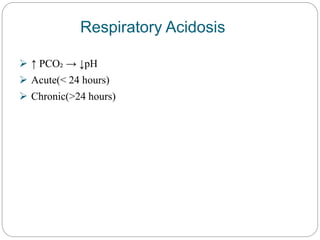
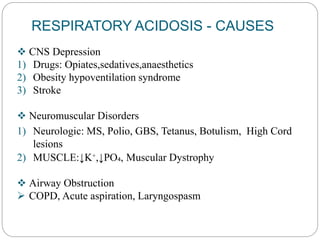
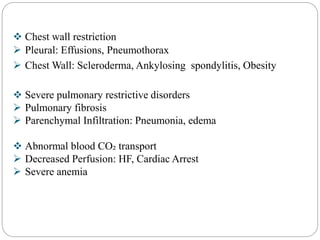
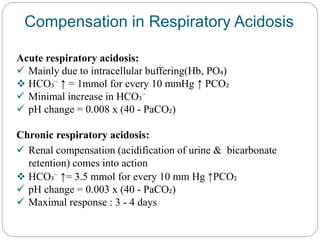
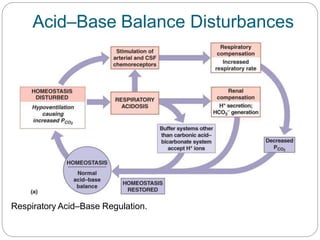
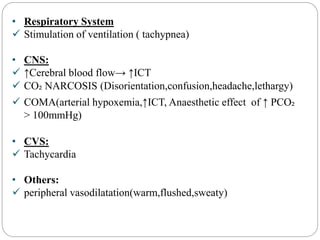
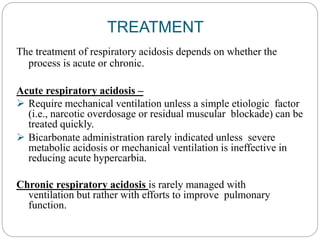
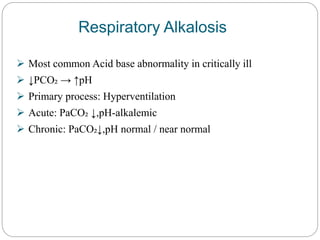
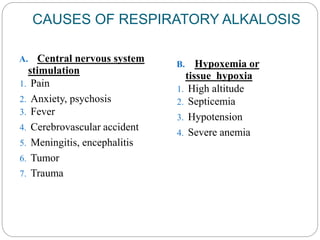
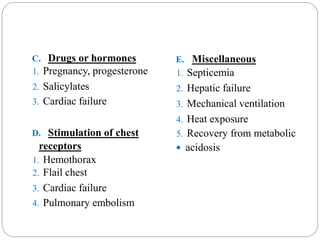
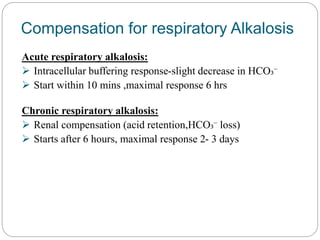
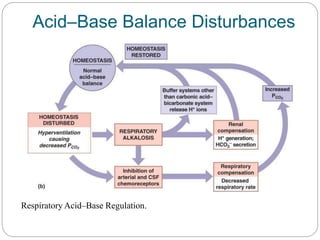
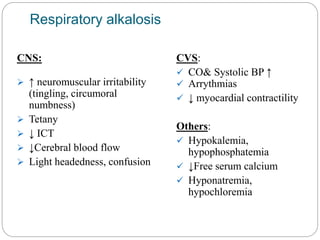
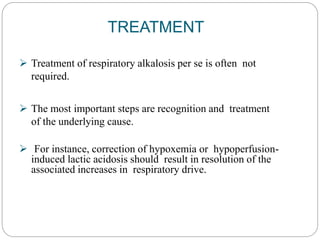
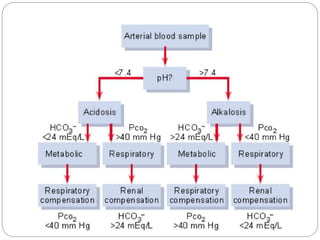
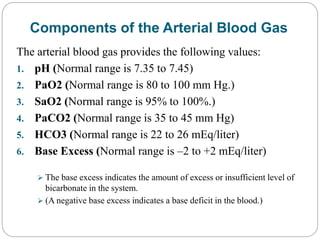
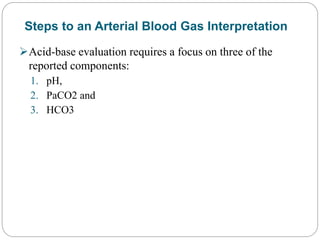
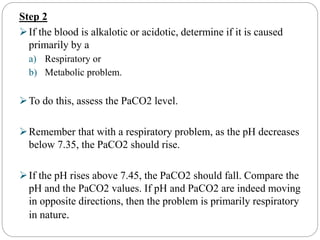
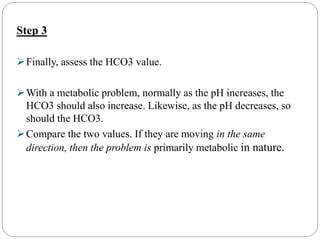
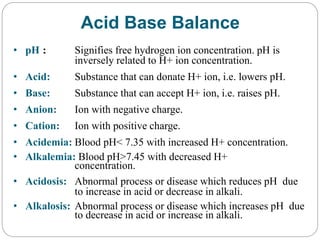
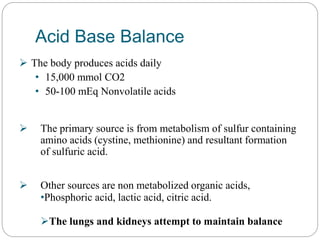
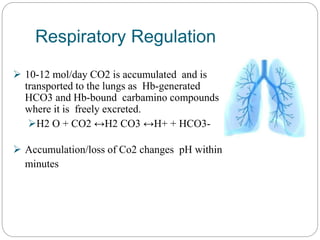
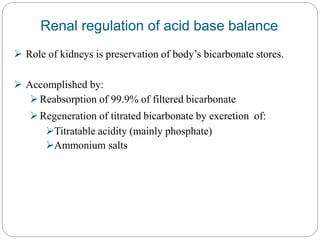
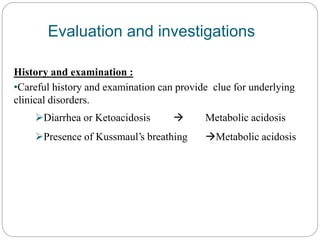
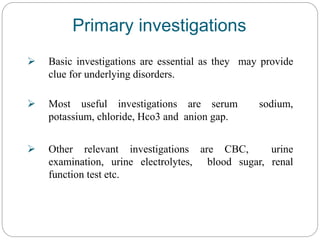
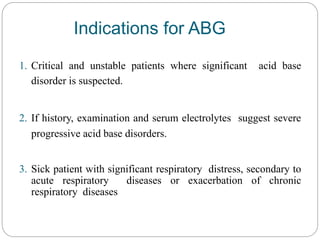
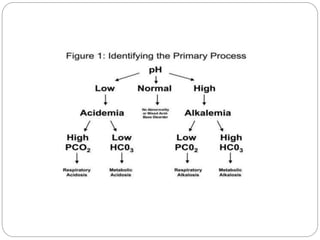
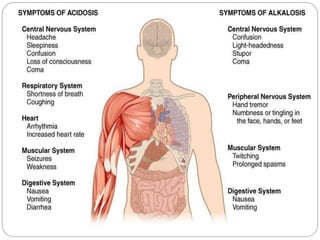
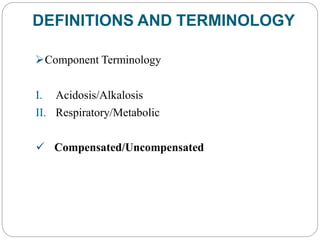
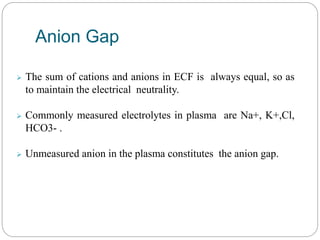
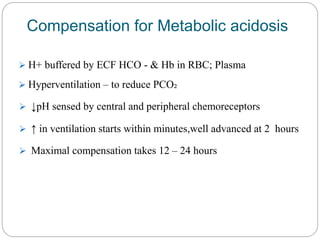
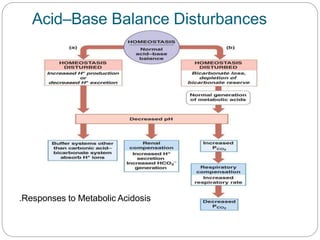
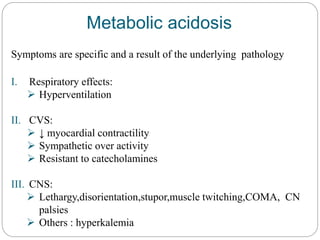
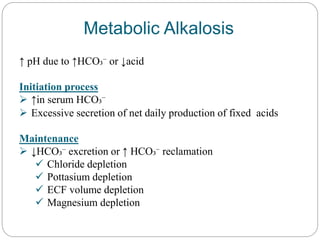
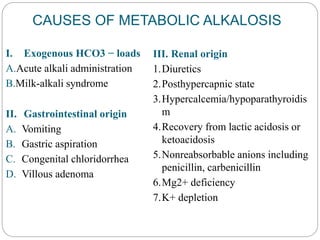
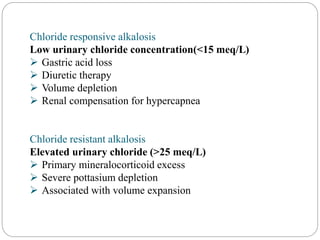
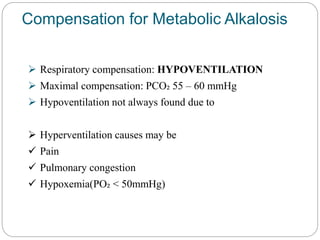
The document provides a comprehensive overview of acid-base balance, including definitions, mechanisms of regulation by the respiratory and renal systems, and descriptions of various acid-base disorders such as acidosis and alkalosis. It emphasizes the importance of maintaining blood pH within the normal range, and details the physiological responses to disturbances, including compensatory mechanisms. Additionally, it discusses diagnostic approaches and treatment options for different acid-base disorders.







![ The initial dose of NaHCO3 can be calculated as:
NaHCO3 (mEq/L)=WT(kgs)x 0.3(24mEq/L-
actual HCO3) / 2
0.3 = the assumed distribution space for bicarbonate and 24
mEq/L is the normal value for [HCO 3-] on arterial blood gas
determination.
The calculation markedly underestimates dosage in severe
metabolic acidosis. In infants and children, a customary initial
dose is 1.0 to 2.0 mEq/kg of body weight.](https://image.slidesharecdn.com/acidbasebalance-230502041449-59ae290d/85/Acid-Base-Balance-pptx-33-320.jpg)
![TREATMENT
Etiologic therapy-
Expansion of intravascular volume or the administration of
potassium.
Infusion of 0.9% saline will dose-dependently increase
serum [Cl-] and decrease serum [HCO3-].
Nonetiologic therapy -
Acetazolamide (a carbonic anhydrase inhibitor that causes
renal bicarbonate wasting)
Infusion of [H+] in the form of ammonium chloride,
arginine hydrochloride, or 0.1 N hydrochloric acid
or dialysis against a high-chloride/low bicarbonate dialysate.](https://image.slidesharecdn.com/acidbasebalance-230502041449-59ae290d/85/Acid-Base-Balance-pptx-38-320.jpg)