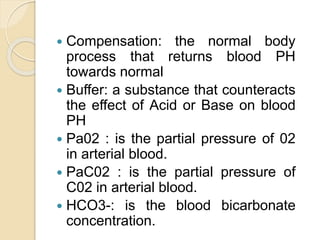
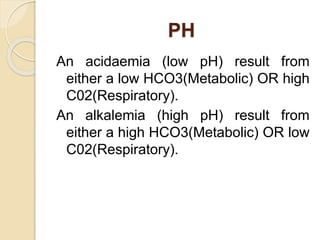
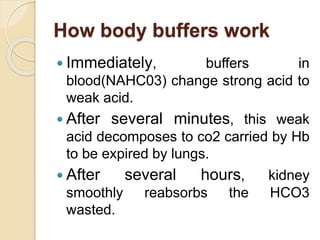
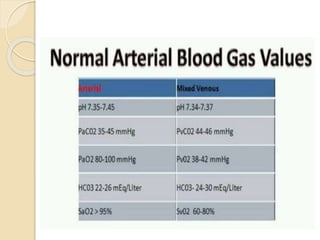
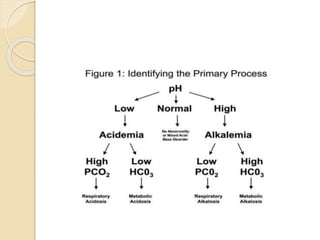
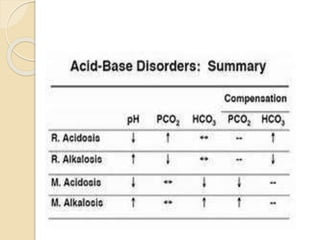
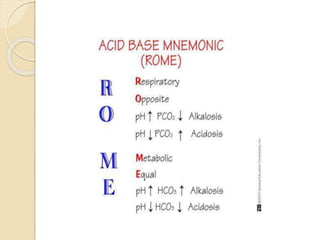
This document defines and describes various acid-base disorders including metabolic and respiratory acidosis and alkalosis. It explains how the body normally buffers changes in blood pH and defines related terms like anion gap. Causes and treatments of different acid-base imbalances are outlined. Proper technique for obtaining arterial blood gas samples is also covered.

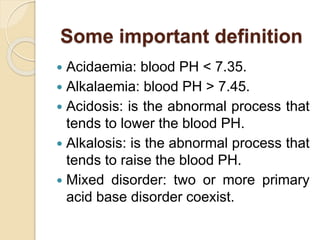

![A-a gradient
A-a gradient= PA02-Pa02
Here, PA02 is alveolar P02 & Pao2 is
arterial P02.
In general, A-a gradient can be calculated
by:
A-a gradient=[Fi02*(Patm-PH20)-
(PaC02/0.8)]-Pao2
On room air & at sea level, Fi02 is
0.21,Patm is 760 mmHg and PH20 is
47 mmHg.](https://image.slidesharecdn.com/acidbasedisorders-180127155928/85/Acid-base-disorders-11-320.jpg)