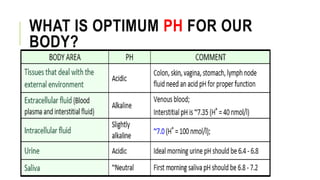
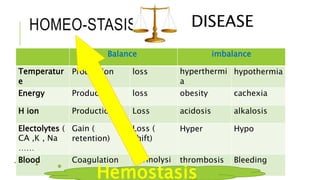
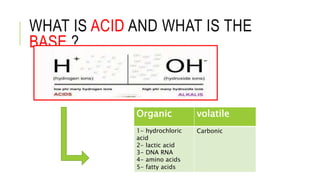
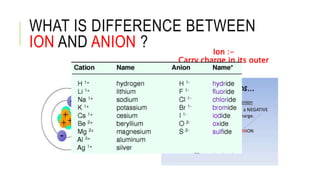
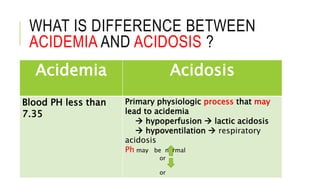
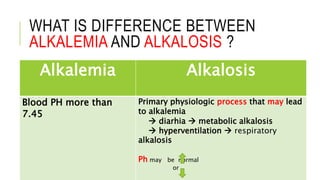
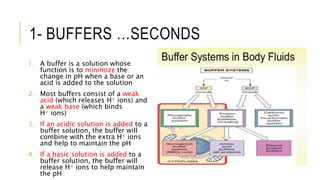
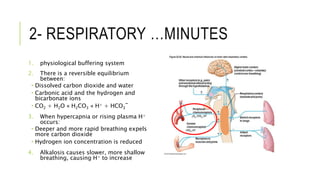
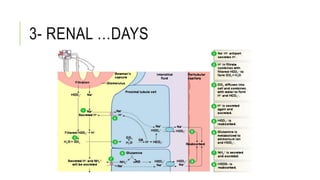
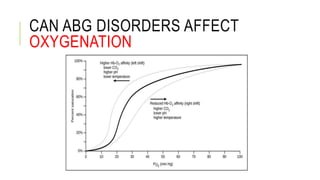
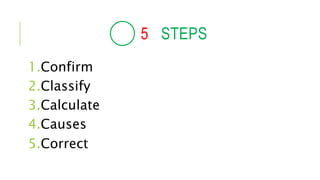
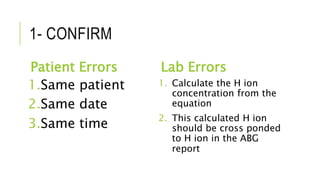
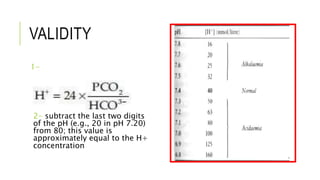
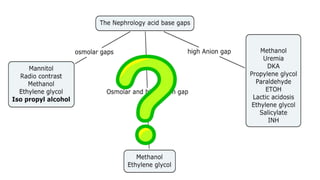
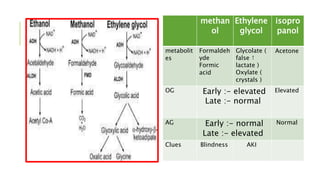
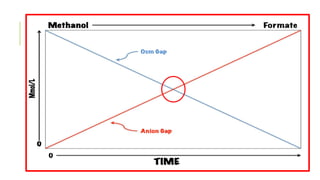
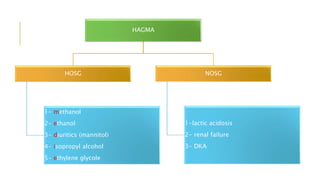
1. The document discusses acid-base balance and arterial blood gases (ABGs), including definitions of pH, the Henderson-Hasselbalch equation, and the three main mechanisms of acid-base regulation: chemical buffers, respiration, and renal.
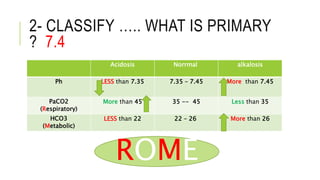
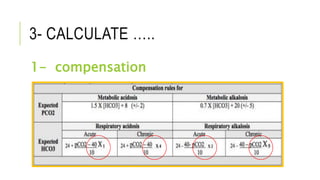
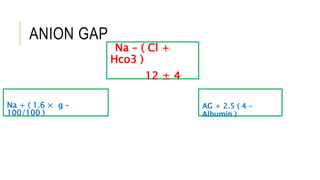
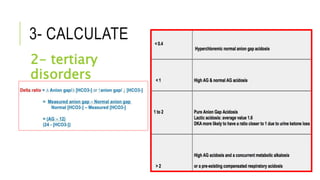
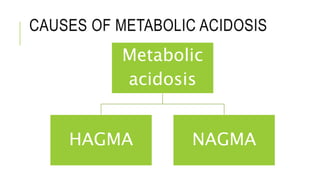
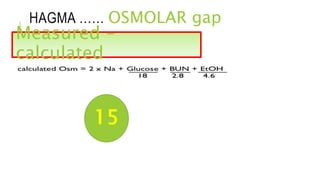
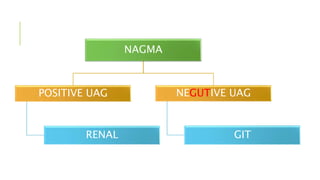
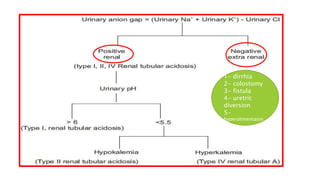
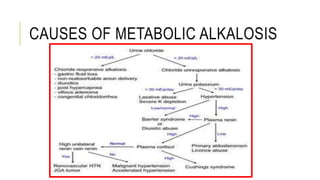
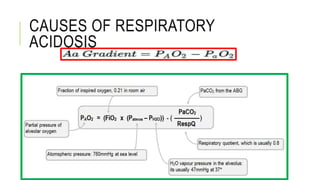
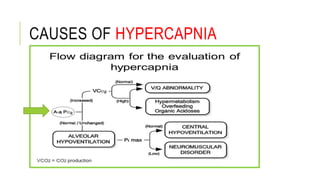
2. It examines the causes, classifications, and compensation mechanisms of metabolic and respiratory acidosis and alkalosis. Mixed acid-base disorders are also addressed.
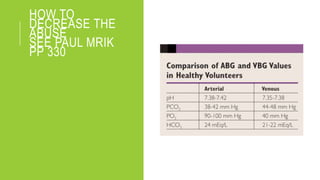
3. The importance of considering the patient's history and clinical presentation when interpreting ABG results is emphasized to help identify underlying etiologies and guide treatment.

![WHAT IS PH ?
pH=-log[H+] concentration,
which is read:
the pH is equal to minus the log of the H+
concentration.
For example is the H+ concentration is very low, lets say
about 0.0000001M, then the pH is
pH= -log[.0000001] whis is the same as -log[1 X 10-7]
the term log[1 X 10-7] = -7
- (-7) = 7](https://image.slidesharecdn.com/abgfinal-160821214451/85/acid-base-ABG-from-theory-to-therapy-2-320.jpg)