The document provides information about arterial blood gas (ABG) analysis, including:
- ABG analysis measures pH, pCO2, pO2, and can determine oxygen saturation. It is commonly performed on critically ill patients.
- The test is used to evaluate gas exchange and acid-base balance. Abnormal results can indicate respiratory or metabolic issues.
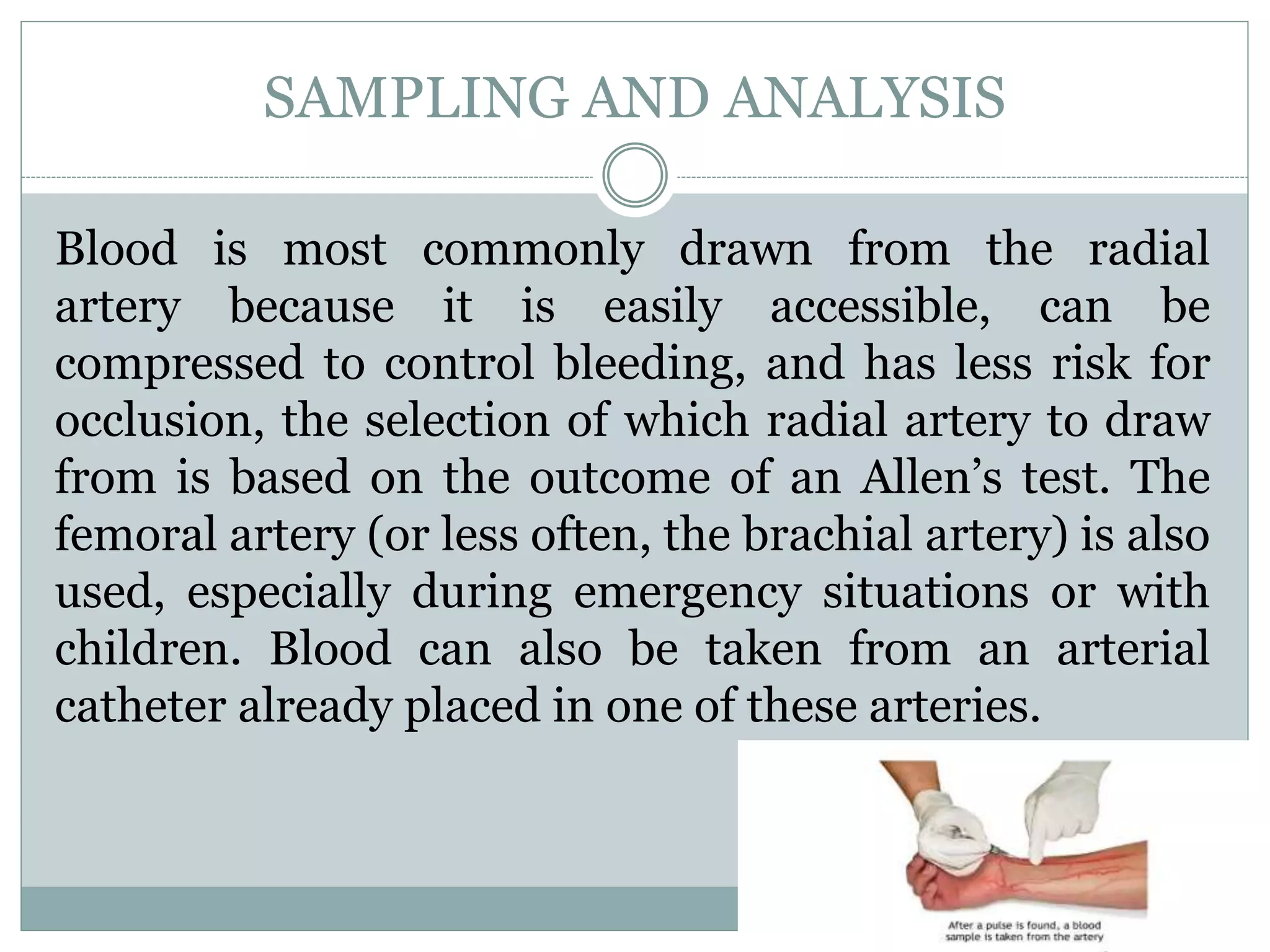
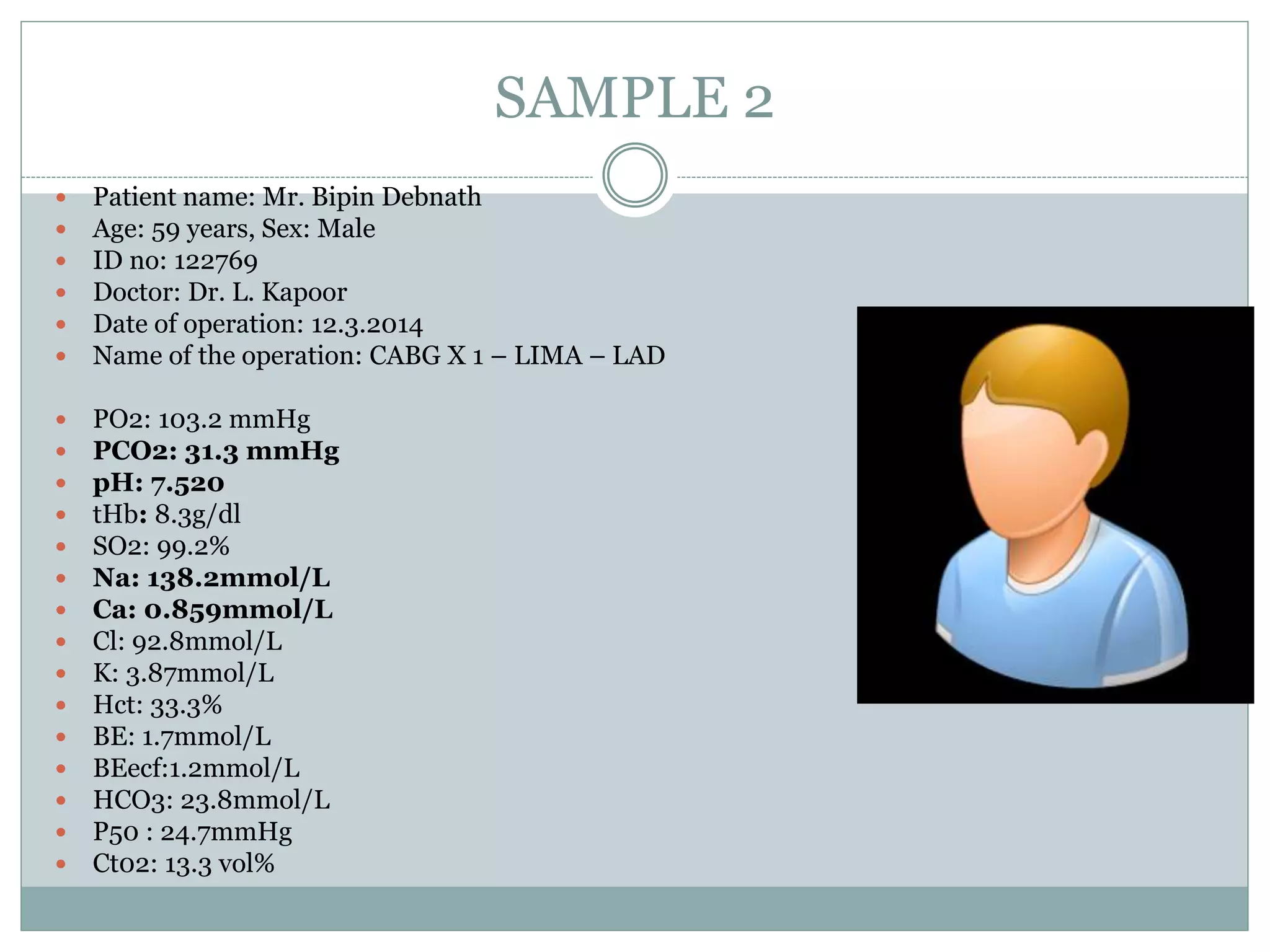
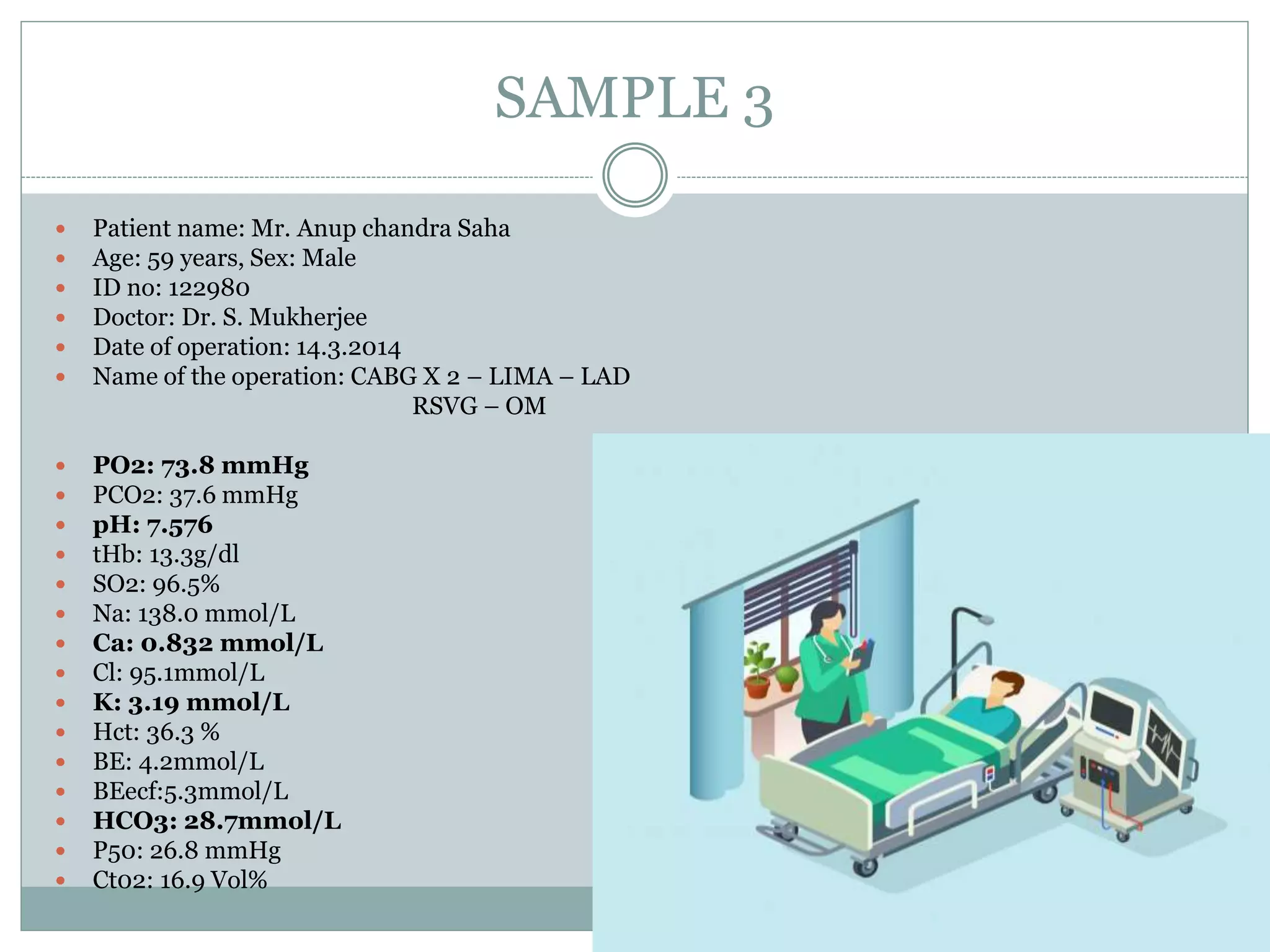
- Proper sampling technique and rapid analysis is important for accurate results. Five sample cases are presented and analyzed.
- Nurses play an important role in understanding ABG results and communicating abnormalities to medical staff for critically ill patients.