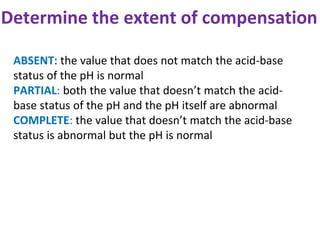
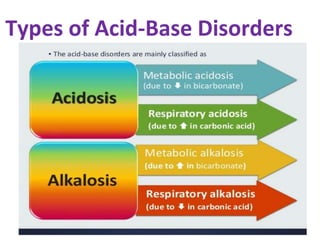
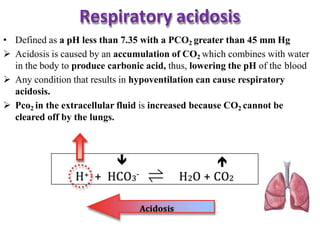
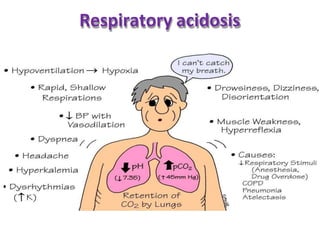
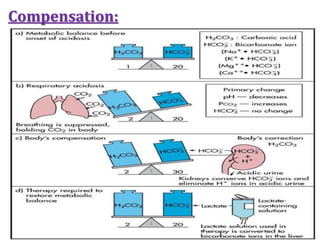
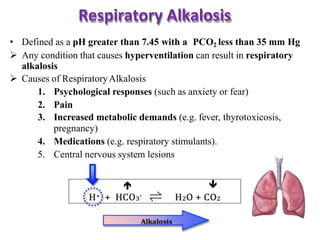
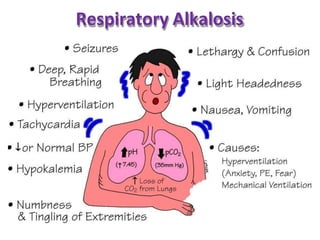
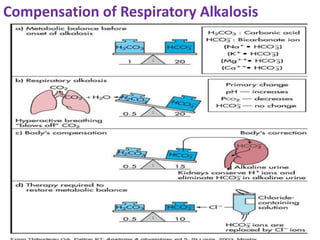
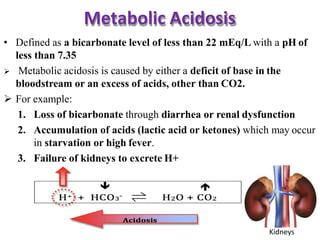
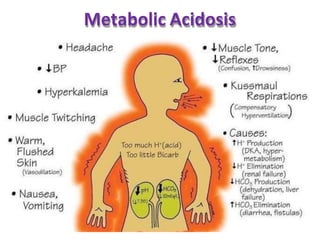
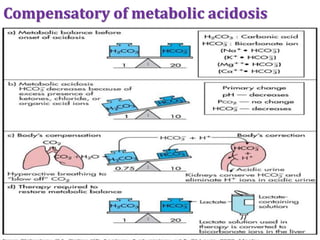
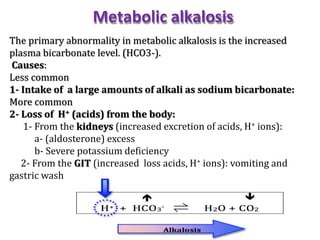
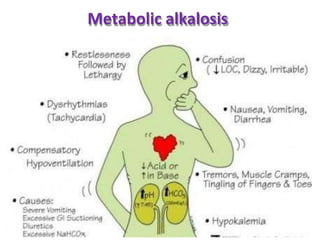
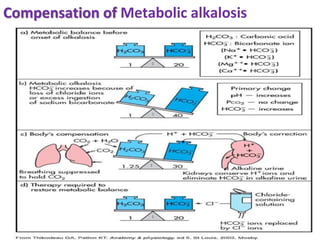
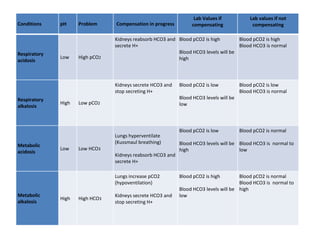
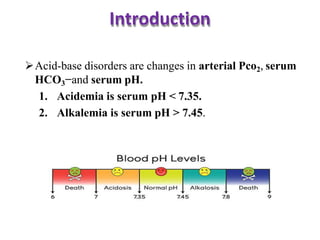
This document discusses acid-base imbalance disorders. It begins by explaining that small pH changes can disrupt enzymes and electrolytes. It then defines acid-base disorders as changes in arterial PCO2, serum HCO3, and pH. The four types of acid-base disorders - respiratory acidosis, respiratory alkalosis, metabolic acidosis, and metabolic alkalosis - are explained in detail. Each disorder section defines the condition, causes, and how the body attempts to compensate through respiratory or renal mechanisms to return blood pH to normal ranges. Compensation is said to be absent, partial, or complete depending on if the compensating value or pH is abnormal.


![Adults Neonates
pH 7.35-7.45 7.30-7.40
pCO2 (kPa)
4.7-6.0 3.5-5.4
Bicarbonate
(mmol/L) 22-28 15-25
pH = 6.1 + log ([HCO3 –] / ( pCO2 (a) × 0.23))
Henderson-Hasselbalch equation](https://image.slidesharecdn.com/acid-basedisorder2020-200913181051/85/Acid-base-imbalance-disorder2020-4-320.jpg)