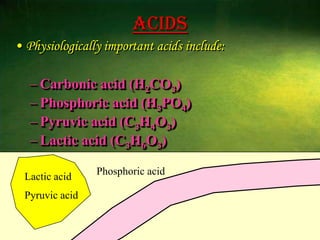
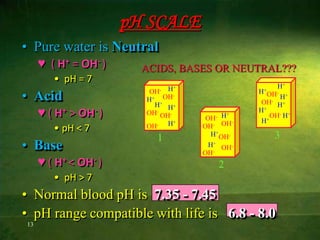
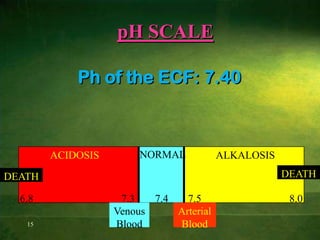
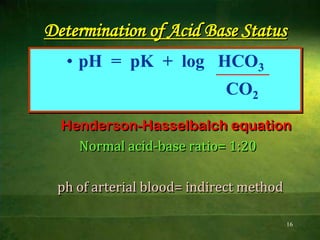
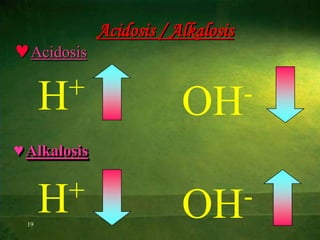
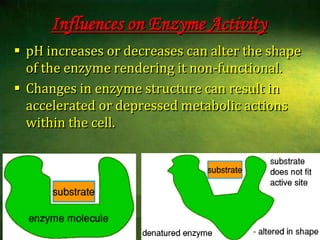
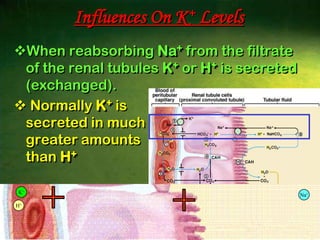
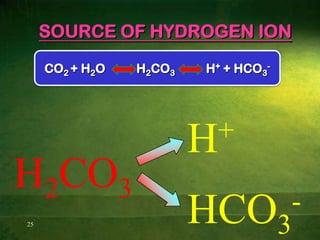
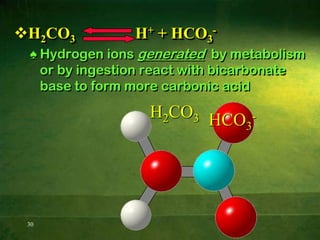
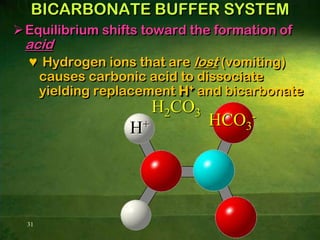
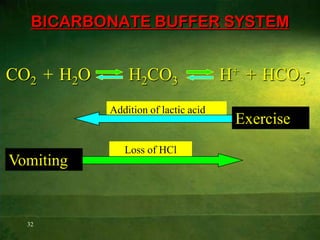
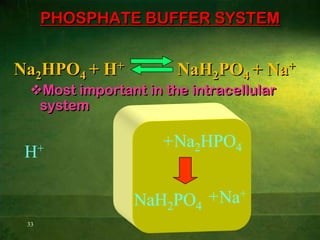
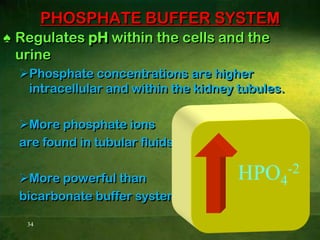
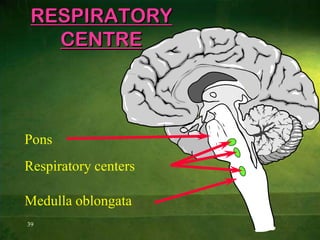
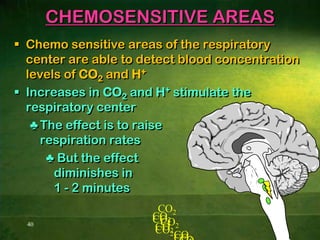
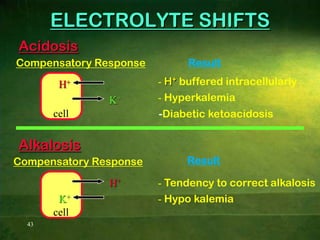
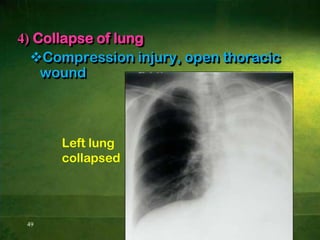
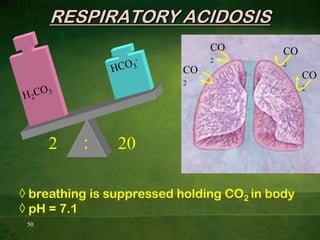
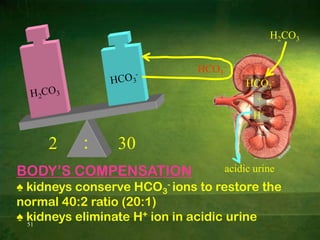
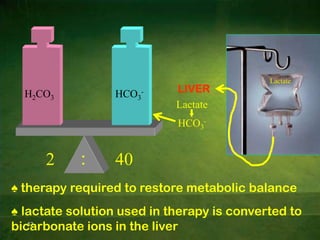
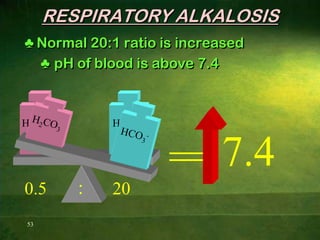
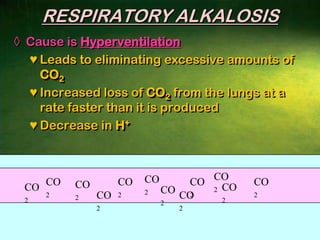
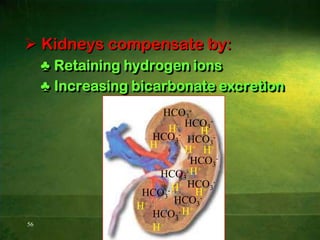
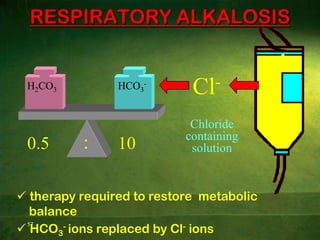
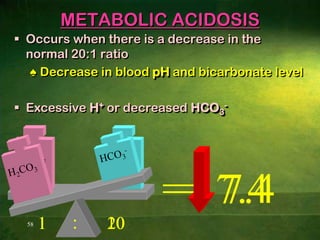
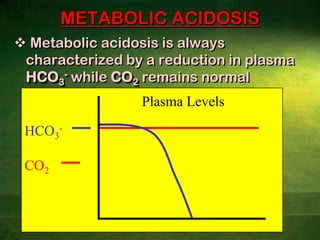
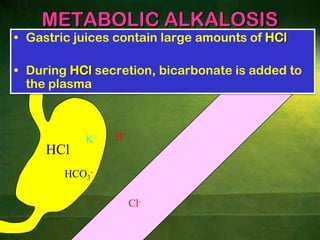
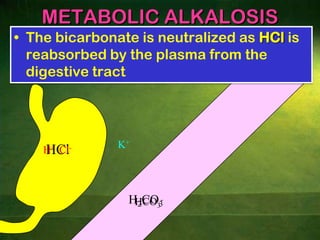
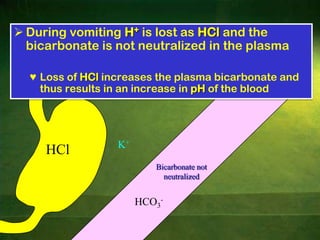
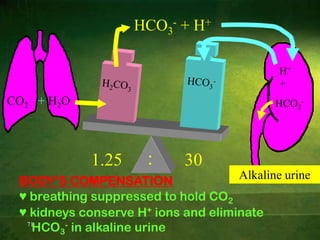
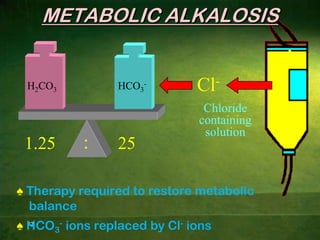
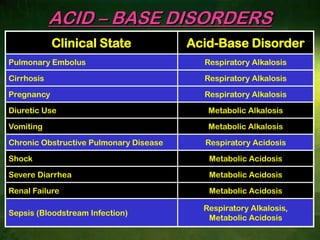
This document discusses physiology of acid-base balance. It defines acids and bases, explains the pH scale and how it relates to acidosis and alkalosis. It describes the major buffer systems that help regulate pH, including the bicarbonate buffer system. Respiratory and renal mechanisms act to compensate for disturbances in acid-base balance through regulating CO2 and bicarbonate levels. Imbalances can be respiratory or metabolic in nature, affecting acidosis or alkalosis.