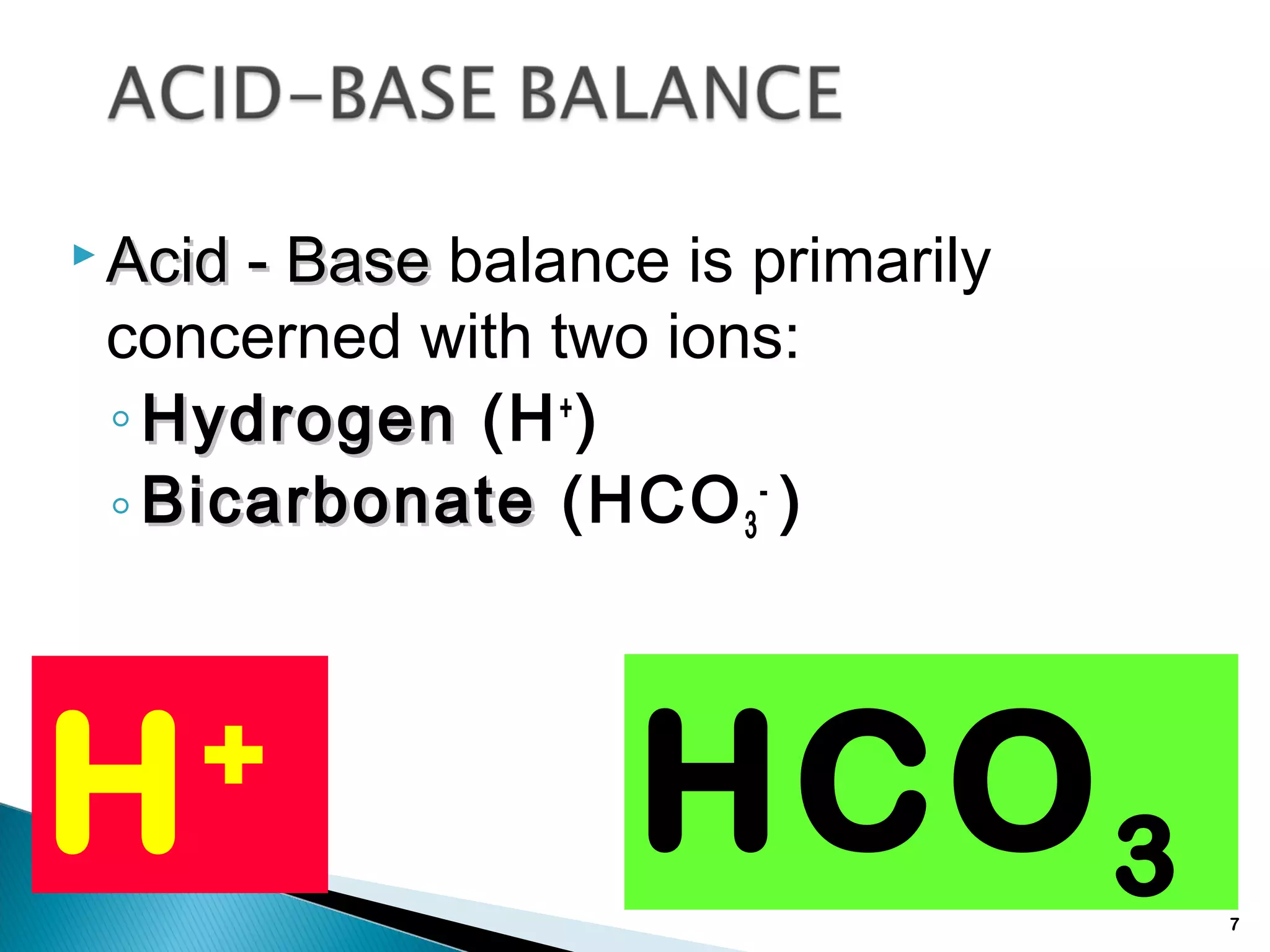
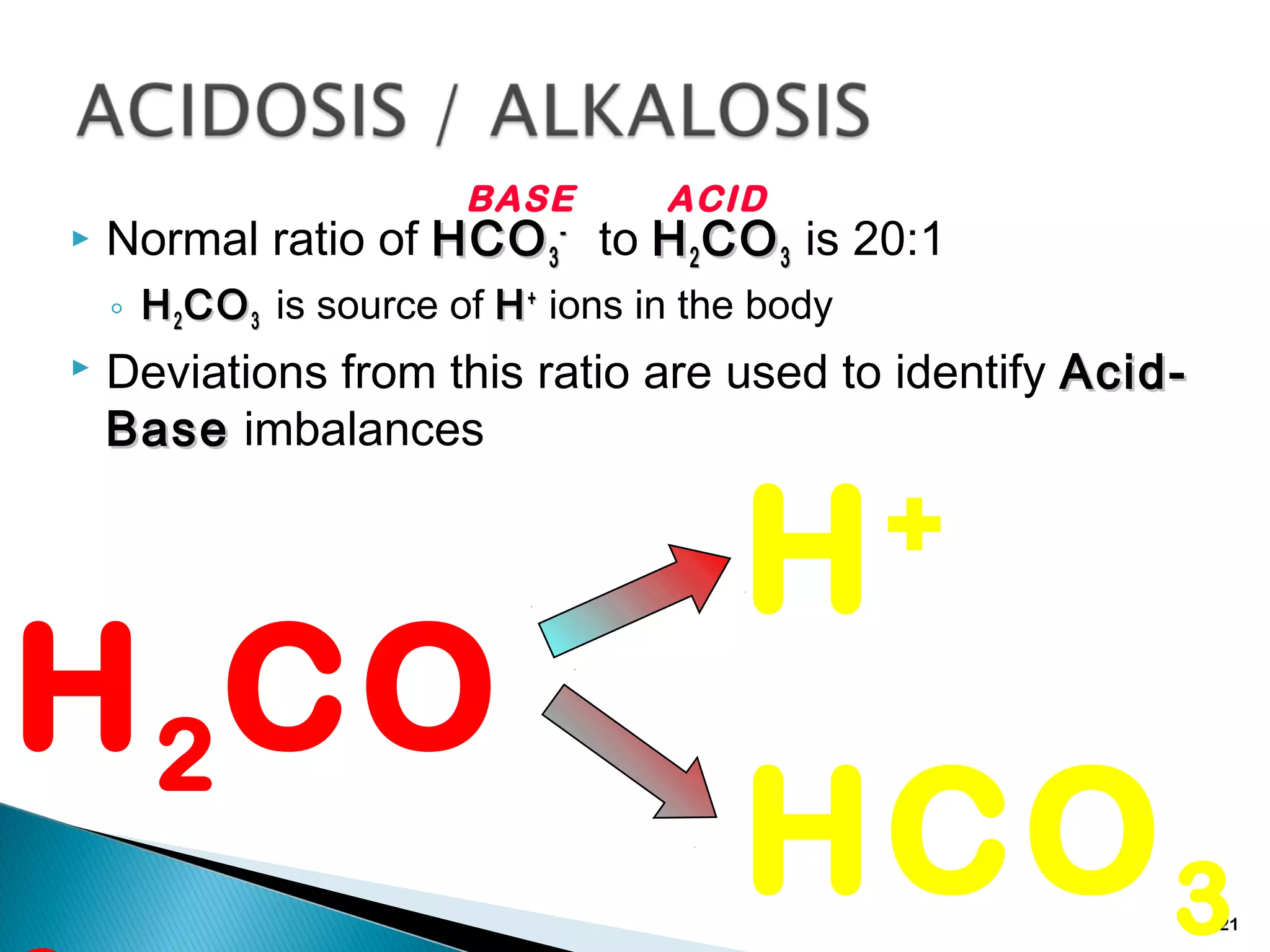
The document discusses acid-base homeostasis and the mechanisms that maintain the pH balance in the body. It covers topics like the importance of pH regulation for cellular functions, the buffers and processes involved in acid-base balance, different types of acid-base imbalances like respiratory acidosis and metabolic alkalosis, and their causes and treatments.