This document discusses acid-base disorders and their physiology, regulation, and treatment. It begins by introducing acid-base balance and pH in the body. It then covers the chemical buffer systems that help regulate pH, as well as the roles of respiration and the kidneys. It discusses different types of acid-base disorders like metabolic acidosis and alkalosis, respiratory acidosis and alkalosis, and mixed disorders. Interpretation of blood gas analysis and various approaches for analyzing acid-base status are also outlined. Throughout, compensation mechanisms and typical treatment approaches for each disorder are described.

![INTRODUCTION
• Blood [H+] is only 0.00004 mEq/L & tightly
controlled
• [H+] is common represented in pH
pH = -log10 [H+]2](https://image.slidesharecdn.com/acid-basedisorders-141008085147-conversion-gate02/85/Acid-base-disorders-2-320.jpg)
![Henderson-Hasselbalch equation
[H+] = (7.80-pH)x100 mEq/L
[H+] in normal pH 7.40 = 40 mEq/L
Respiration
Renal](https://image.slidesharecdn.com/acid-basedisorders-141008085147-conversion-gate02/85/Acid-base-disorders-9-320.jpg)
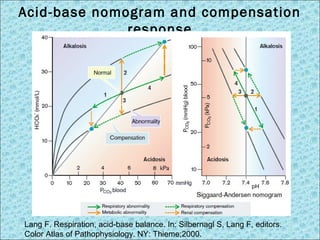
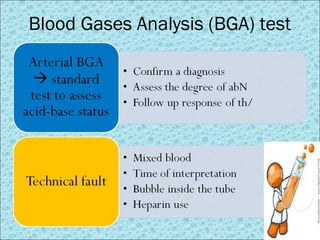
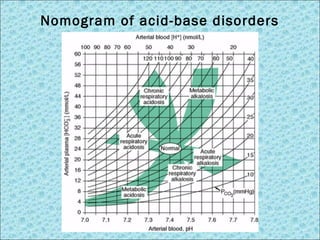
![Interpretation of BGA
Oxygenation status Acid-base status
pH correlated to [H+] acid-base degree
pCO2 Oxygen partial pressure in blood; normal 80-100 mmHg
SaO2 Arterial oxygen saturation; normal 95-100%
pCO2 CO2 partial pressure in blood; normal 35-45 mmHg
HCO3- Bicarbonate in circulation (calculated); normal 22-26
mEq/L
Base Excess (BE) Deficit or excess of bicarbonate in blood; normal -2 to +2
mEq/L
Parameters in BGA test](https://image.slidesharecdn.com/acid-basedisorders-141008085147-conversion-gate02/85/Acid-base-disorders-14-320.jpg)
![Stewart’s SID
• Dependent variables: [H+], [OH-], [HCO3-], [CO3 2-], [HA],
[A-]
• Independent variables: pCO2, [A-tot], [SID]
– [SID]= [Na]+[K]+[Ca]+[Mg] – [Cl] – [other strong anions]
normal = 40 mEq/L similar numerical value as BE
– [A-tot]= [Pi-tot] + [Pro-tot] + albumin
– [CO2] = pCO2 in blood
• Limitation:
– Complexity of the chemistry & mathematics
– Lack of clinical correlation
– SID neglects Hb as a buffer less accurate than BE
www.acid-base.com/strongion.php](https://image.slidesharecdn.com/acid-basedisorders-141008085147-conversion-gate02/85/Acid-base-disorders-18-320.jpg)
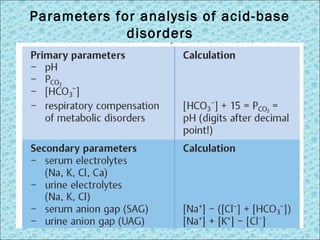
![Interpretation of Handerson Hasselbalch approach
STEP 1 Re-check the data [H+]=24 x pCO2 / [HCO3-]
[H+] mEq/L= (7.80-pH)x100
STEP 2 Acidemia (pH <7.37) or alkalemia (pH
>7.42) ?
STEP 3 Determining the primary disorder; metabolic or
respiratory ? look any Δ pCO2 and HCO3-
STEP 4 Determining compensatory mechanisms expected
or excessive deviation ?
STEP 5
(for metab acidosis)
Anion Gap= [Na]- ( [Cl] + [HCO3])…...... n<12
Hypoalbuminemia AGc= AG + (2.5x Δ albumin)
STEP 6
(for AG > 12)
Delta/delta = ΔAG/ΔHCO3 = (AG-12)/(24-HCO3)
Delta/delta > 1 = metab acidosis + alkalosis
Delta/delta < 1 = metab.acidosis gap + non-gap](https://image.slidesharecdn.com/acid-basedisorders-141008085147-conversion-gate02/85/Acid-base-disorders-19-320.jpg)

![Therapy of metabolic
acidosis
• Correct underlying disease
• Correct hydration state and electrolyte imbalance
• Bicarbonate controversial
- Indicated for severe acidosis (pH < 7.20), esp.
GAP metabolic acidosis
- total needed (mEq)= Base deficit x BW(kg)/4 ½ doses is given
within first 8 h
• Chronic non-severe acidosis: bicarbonate oral for
[HCO3-] <18 mmol/L + clinical symptoms](https://image.slidesharecdn.com/acid-basedisorders-141008085147-conversion-gate02/85/Acid-base-disorders-23-320.jpg)

![Type and therapy of metabolic
alkalosis
Chloride-sensitive Chloride-insensitive
* [Cl-] urine < 10 mEq/L
* prolonged Cl (and H+) loss
via urine/GI tract Na and
HCO3 retention by renal
* GI tract loss: vomit, NGT
suction, diarrhrea
* renal loss: diuretic
* response to NaCl therapy
• [Cl-] > 10 mEq/L
• direct stimulation to renal
• causa: hyperaldosteronism,
steroid therapy, alkali intake
• not response to NaCl
therapy; therapy focused on
underlying cause (ex. stop
consuming steroid)
Therapy with strong acid (HCl, NH4Cl) is only for severe alkalosis and
resistant with standard therapy](https://image.slidesharecdn.com/acid-basedisorders-141008085147-conversion-gate02/85/Acid-base-disorders-25-320.jpg)