This document summarizes acid-base homeostasis and disorders. It discusses how the body maintains pH between 7.35-7.45 through three main systems: buffers, respiration, and the kidneys. Buffers act quickly to reduce free hydrogen ions while respiration and the kidneys provide longer-term regulation. Disorders occur when pH falls outside the normal range due to low or high blood bicarbonate levels or carbon dioxide levels. The document outlines the causes, signs, and treatments of various acid-base imbalances.

![Acid-Base Balance
• Normal pH: 7.4 [ H⁺ : 40 nmol/L]
• Body fluids maintained pH between 7.35-7.45
• Clinically safe: 7.3-7.5
• Compatible to life: 6.8-7.8
• Recovery unusual: <6.8 & >7.8
• Low pH: Acidic
• High pH: Alkaline](https://image.slidesharecdn.com/acidbasehomeostasis-210120142411/75/Acid-base-homeostasis-2-2048.jpg)
![Origin of acid
Volatile acid [H₂CO₃]
• Aerobic metabolism of carbohydrate & lipid
Non volatile acid [all except H₂CO₃]
• Anaerobic metabolism of carbohydrate: lactic acid
• Insulin lack: Ketoacids
• Diet (protein)
HCl
Phosphoric acid- arginine, histidine
Sulfuric acid- cystine, methionine](https://image.slidesharecdn.com/acidbasehomeostasis-210120142411/75/Acid-base-homeostasis-3-2048.jpg)





![Compensation & Correction of ABD
• How this ratio is maintained?
1.By increasing/decreasing
plasma [HCO₃⁻]
2.By reducing/increasing PCO₂
• Normal [HCO₃⁻]:[H₂CO₃] =
20:1
• Compensation:
Maintaining the ratio of
[HCO₃⁻]:[H₂CO₃] towards
normal, so pH become
normal
• Correction: Concentration
of [HCO₃⁻] & [H₂CO₃]
become normal.](https://image.slidesharecdn.com/acidbasehomeostasis-210120142411/75/Acid-base-homeostasis-9-2048.jpg)
![Respiratory Mechanism for pH Regulation
• Rapid but short term regulation
• Regulate [H₂CO₃] concentration in blood
H₂CO₃ CO₂ + H₂O
• All CO₂ is eliminated via the lungs
Carbonic Anhydrase](https://image.slidesharecdn.com/acidbasehomeostasis-210120142411/75/Acid-base-homeostasis-10-2048.jpg)




![Simple Method to Diagnose ABD
Look at the pH
• pH <7.35 (acidosis); pH >7.45 (alkalosis)
Is the CO₂ abnormal?
• CO₂ is an acidic gas; raised with acidosis, lowered with
alkalosis Respiratory problem
• No change; or an opposite one ↑↑↓↓ compensatory change
Is the HCO₃⁻ abnormal? [Normal: 22-28mmol/L]
• Is the change in keeping with pH?
• HCO₃⁻ is alkaline; raised with alkalosis, lowered with
acidosis Metabolic problem](https://image.slidesharecdn.com/acidbasehomeostasis-210120142411/75/Acid-base-homeostasis-15-2048.jpg)

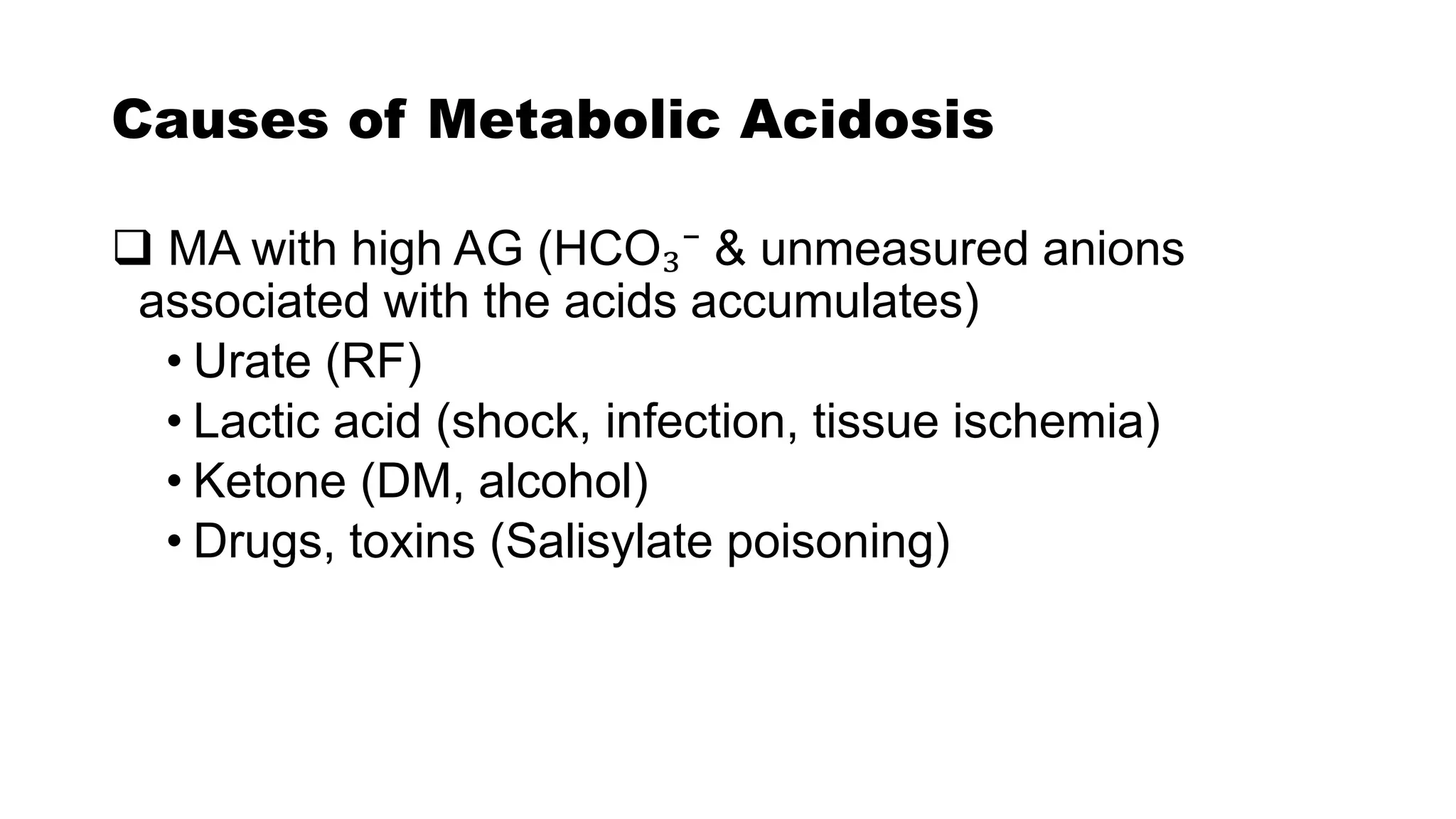
![Causes of ABD
Metabolic acidosis
[↑ production /↓ removal of
fixed or organic acid]
Metabolic alkalosis
[Accumulation of base/ loss of
acid other than H₂CO₃]
• Diabetic ketoacidosis
• Acute MI
• Lactic acidosis
• CRF
• Renal tubular acidosis
• Watery diarrhea
• Intestinal fistula
• Vomiting
• K depletion (diuretics)
• Burns
• Ingestion of base
• Excessive infusion of NaHCO₃](https://image.slidesharecdn.com/acidbasehomeostasis-210120142411/75/Acid-base-homeostasis-17-2048.jpg)
![Causes of ABD
Respiratory acidosis
[Impaired excretion of CO₂]
Respiratory alkalosis
[excessive ventilation]
• COPD
• Chronic bronchitis
• Pulmonary fibrosis
• Acute bronchial asthma
• Narcotic overdose
• Anesthesia
• Stroke
• SAH
• Meningitis
• High altitude
• Hysteria
• Fever
• Pulmonary emboli
• Salicylate poisoning
• Pregnancy](https://image.slidesharecdn.com/acidbasehomeostasis-210120142411/75/Acid-base-homeostasis-18-2048.jpg)