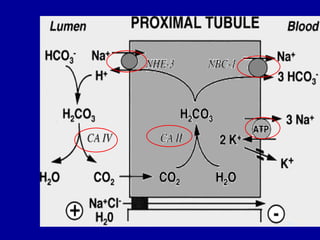
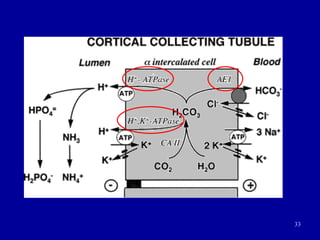
This document discusses acid-base balance in the human body. It defines acids and bases, and explains how the body tightly regulates hydrogen ion concentration through multiple buffer systems including bicarbonate, proteins, and hemoglobin. The lungs and kidneys play key roles in excretion and reabsorption of ions to maintain homeostasis. Respiratory and metabolic acidosis and alkalosis can occur if these control mechanisms are impaired due to conditions like kidney failure or respiratory disease.