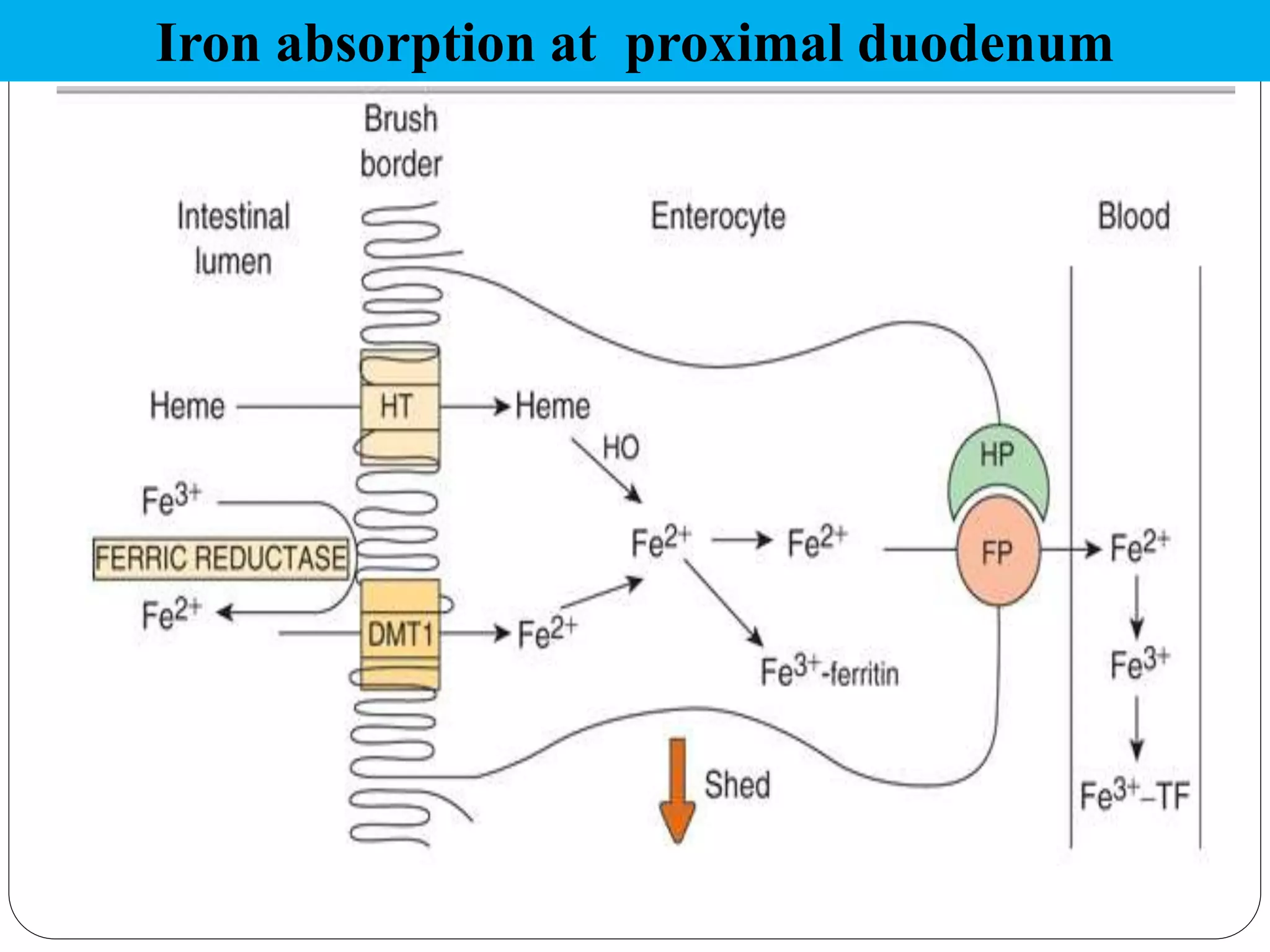
Iron is an essential micronutrient, but both iron deficiency and excess can be harmful. Iron deficiency anemia affects 65-75% of people in India and can impact growth and development. The body tightly regulates iron levels through absorption in the duodenum, transport by transferrin, and storage in ferritin and hemosiderin. Hepcidin is the key regulator of iron absorption and release, inhibiting the iron exporter ferroportin. Disorders of iron metabolism include iron deficiency anemia, hemosiderosis, and hereditary hemochromatosis.