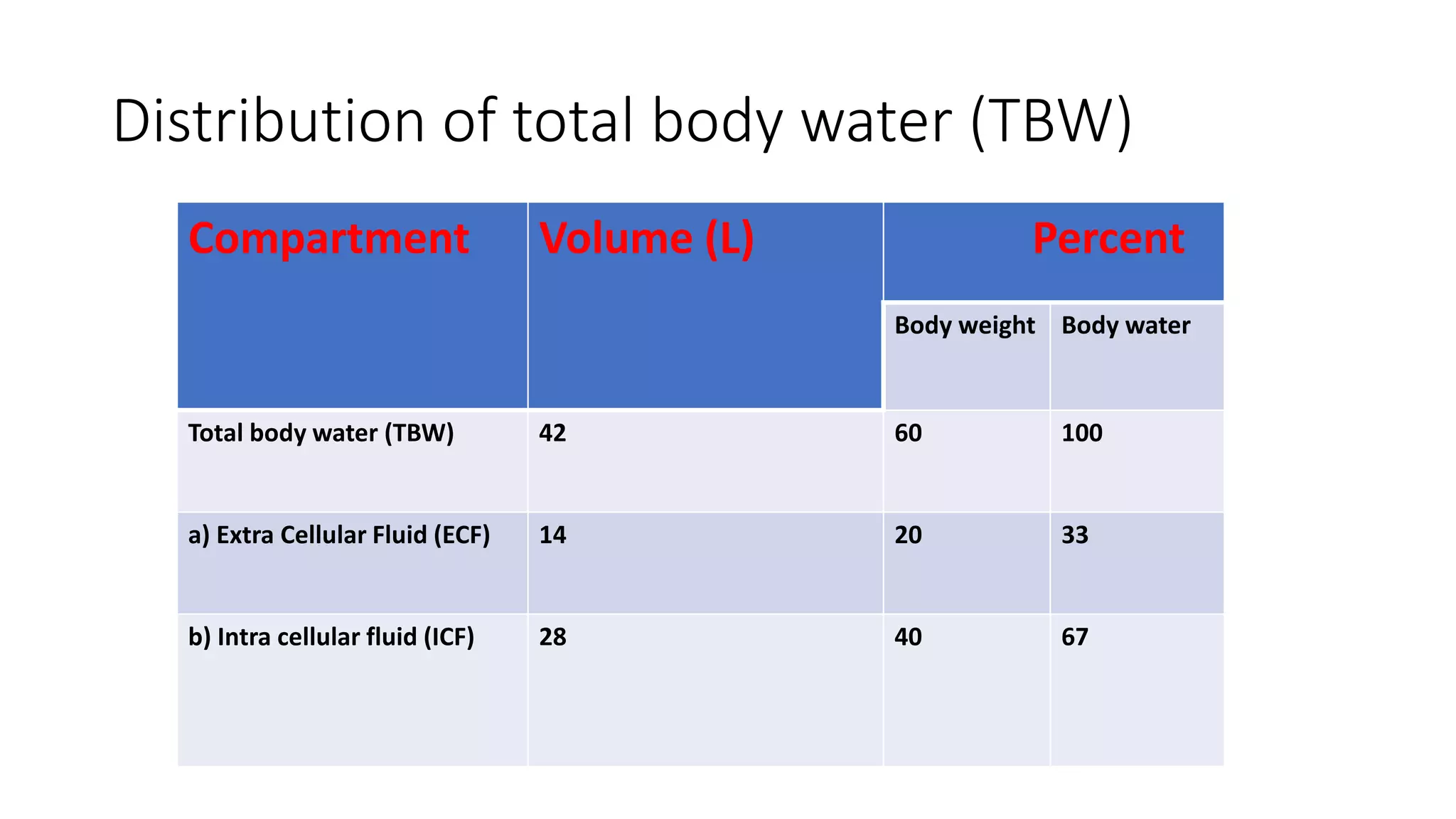
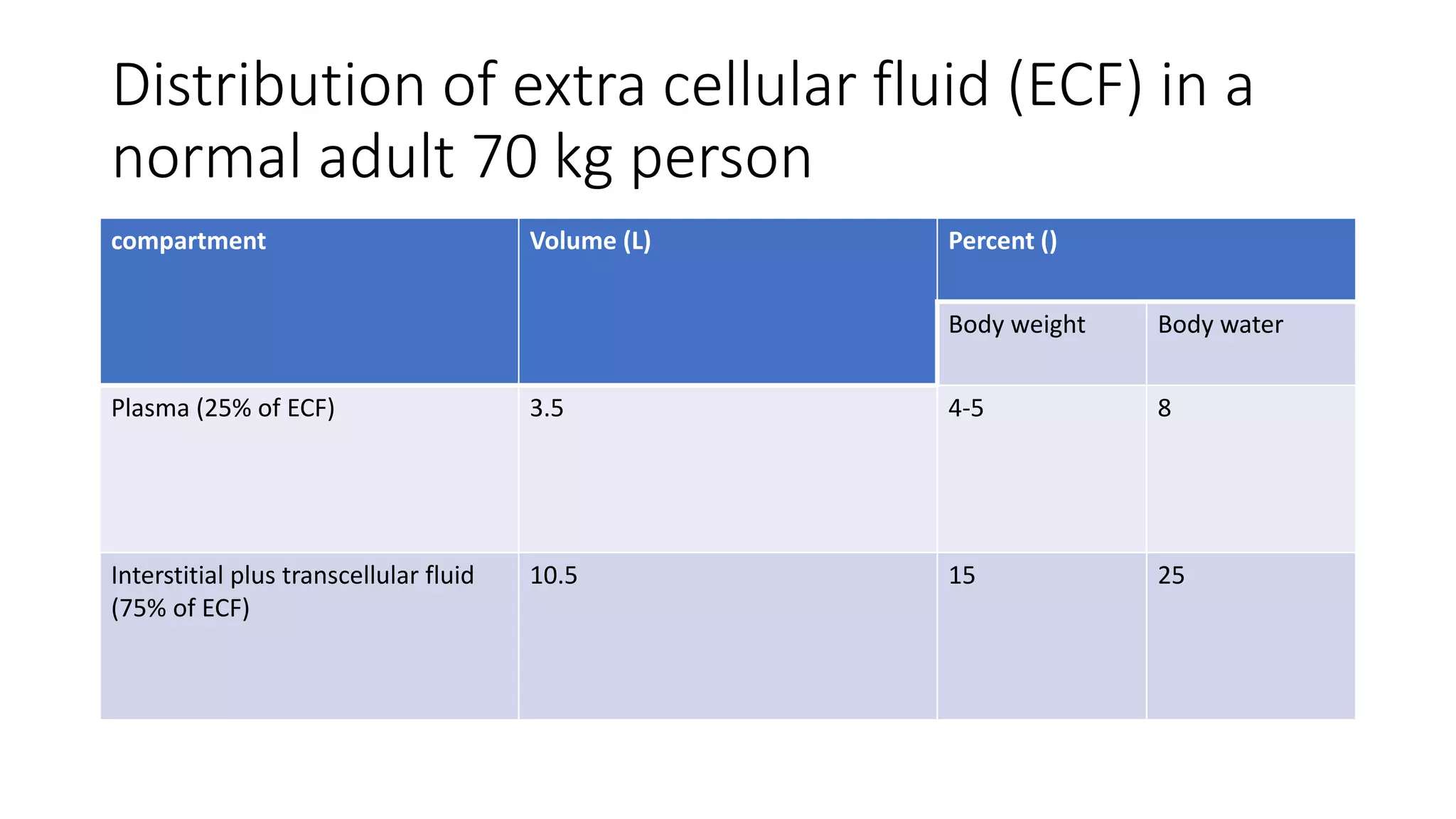
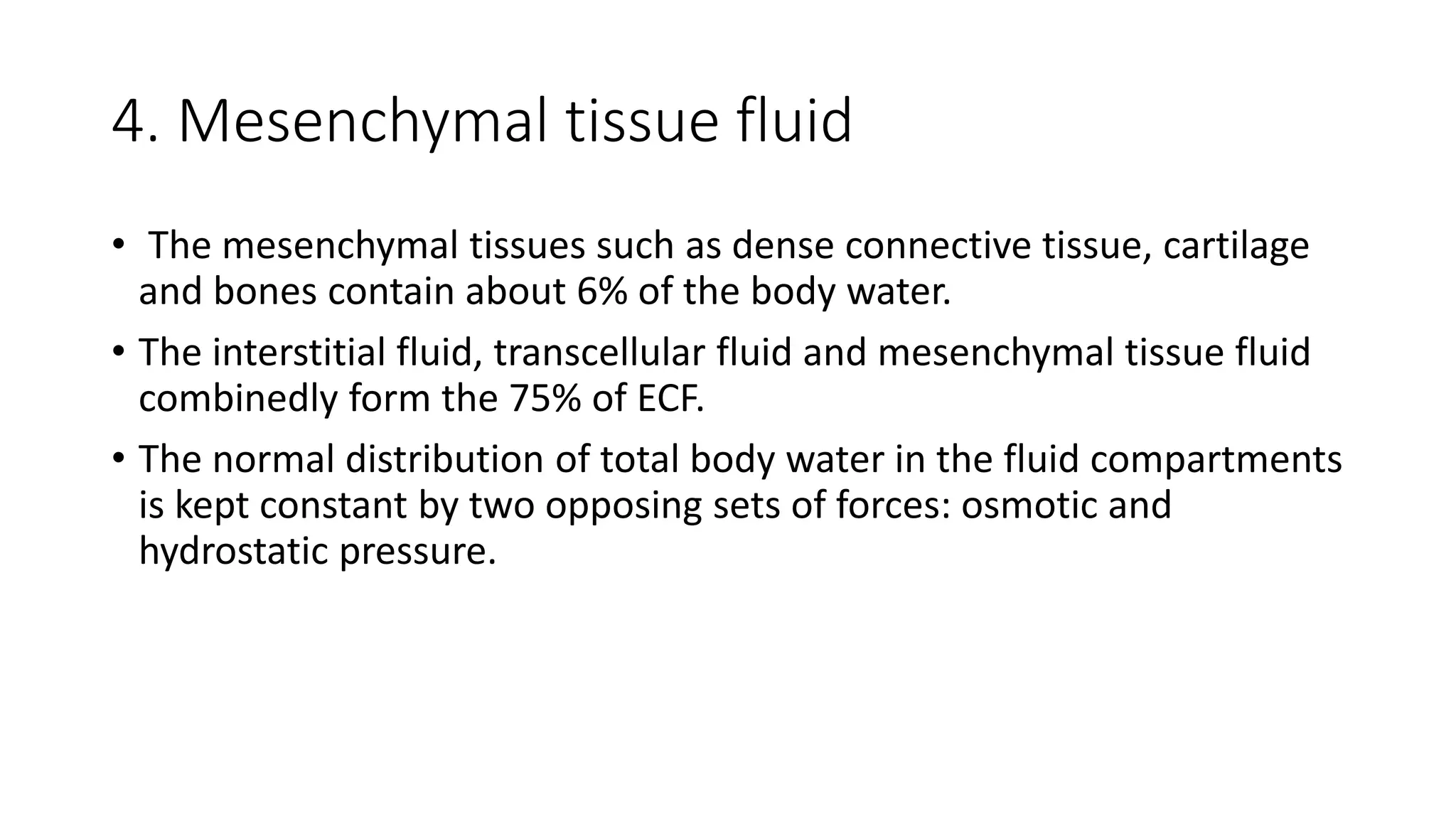
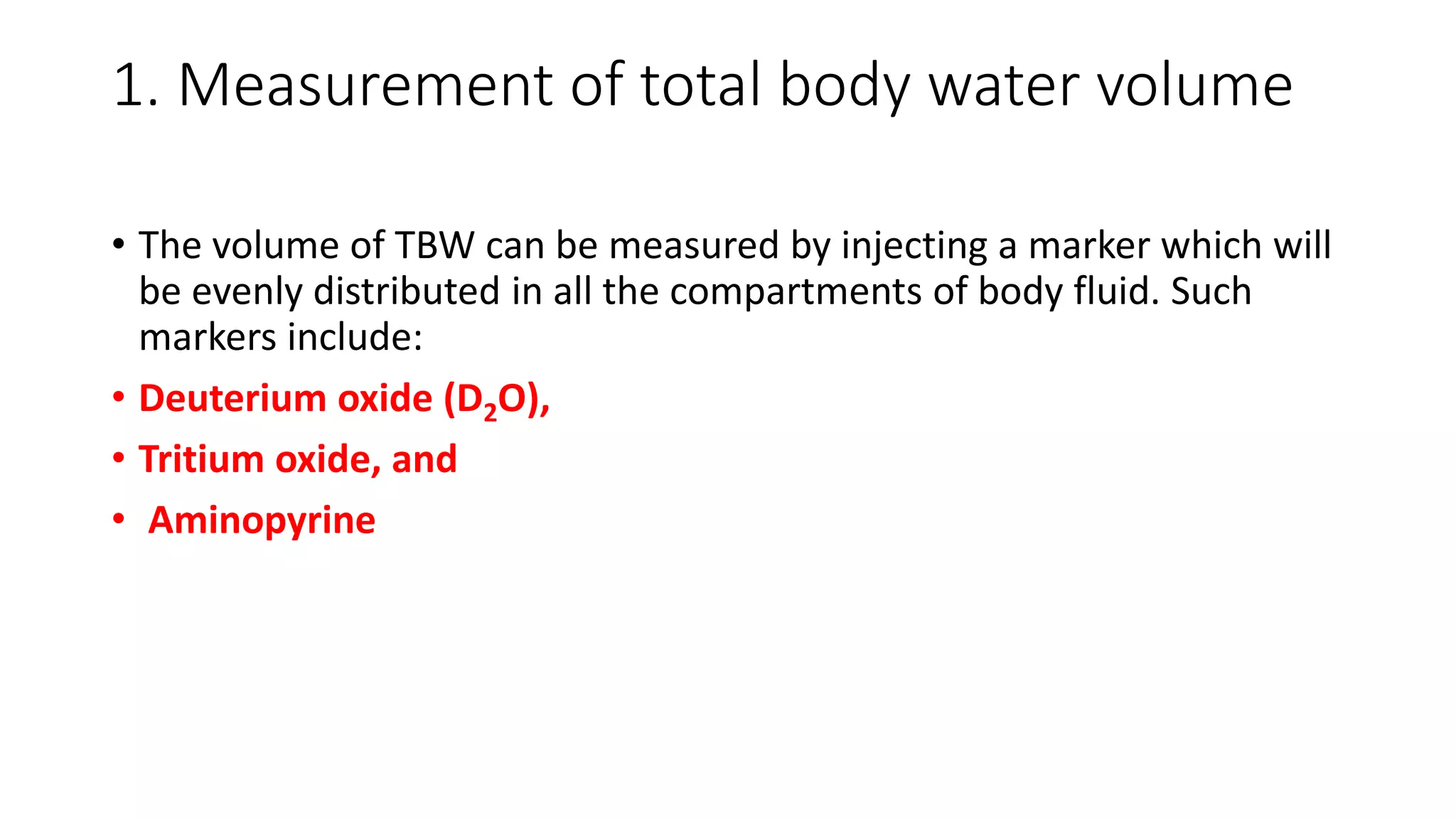
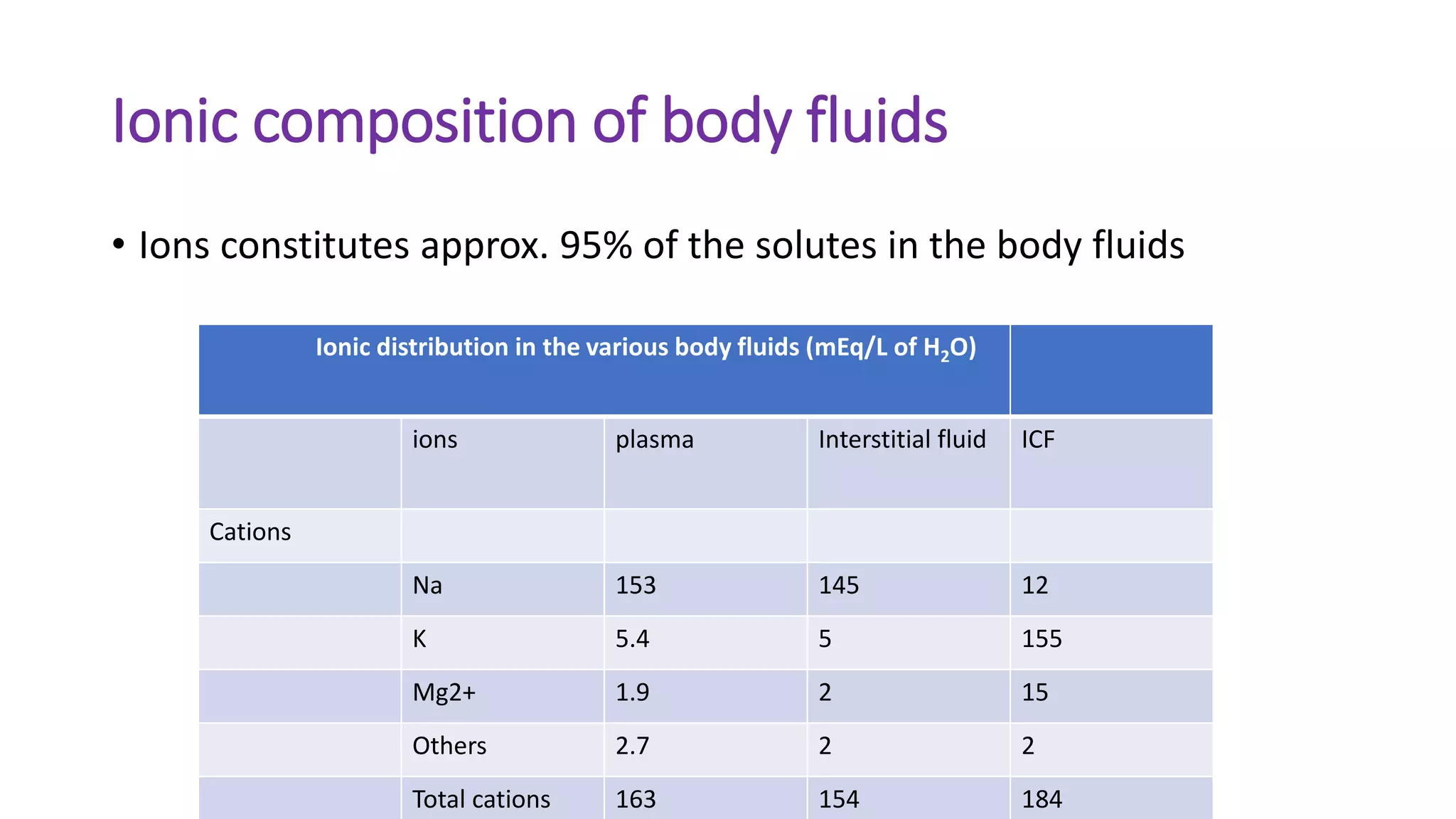
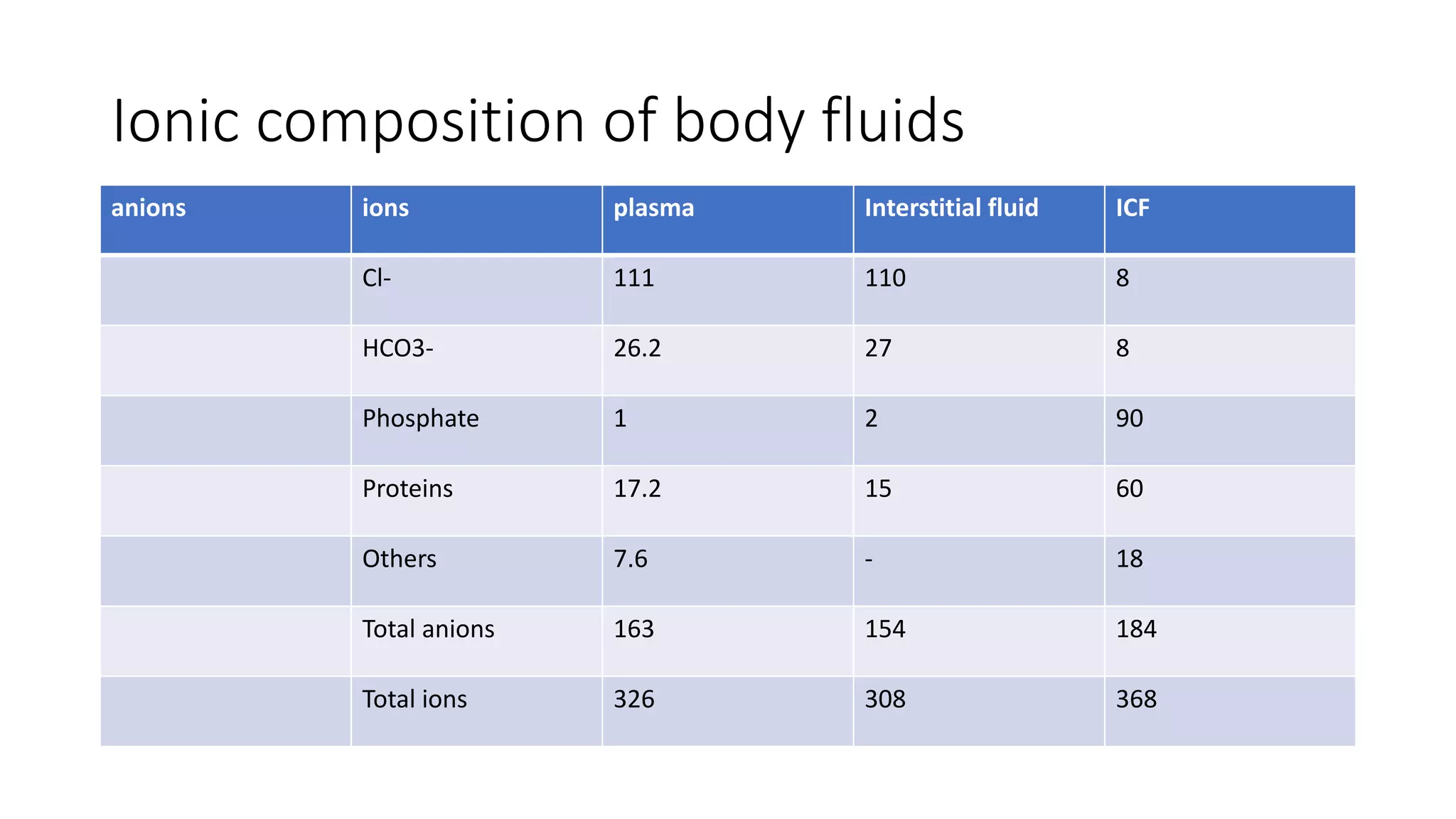
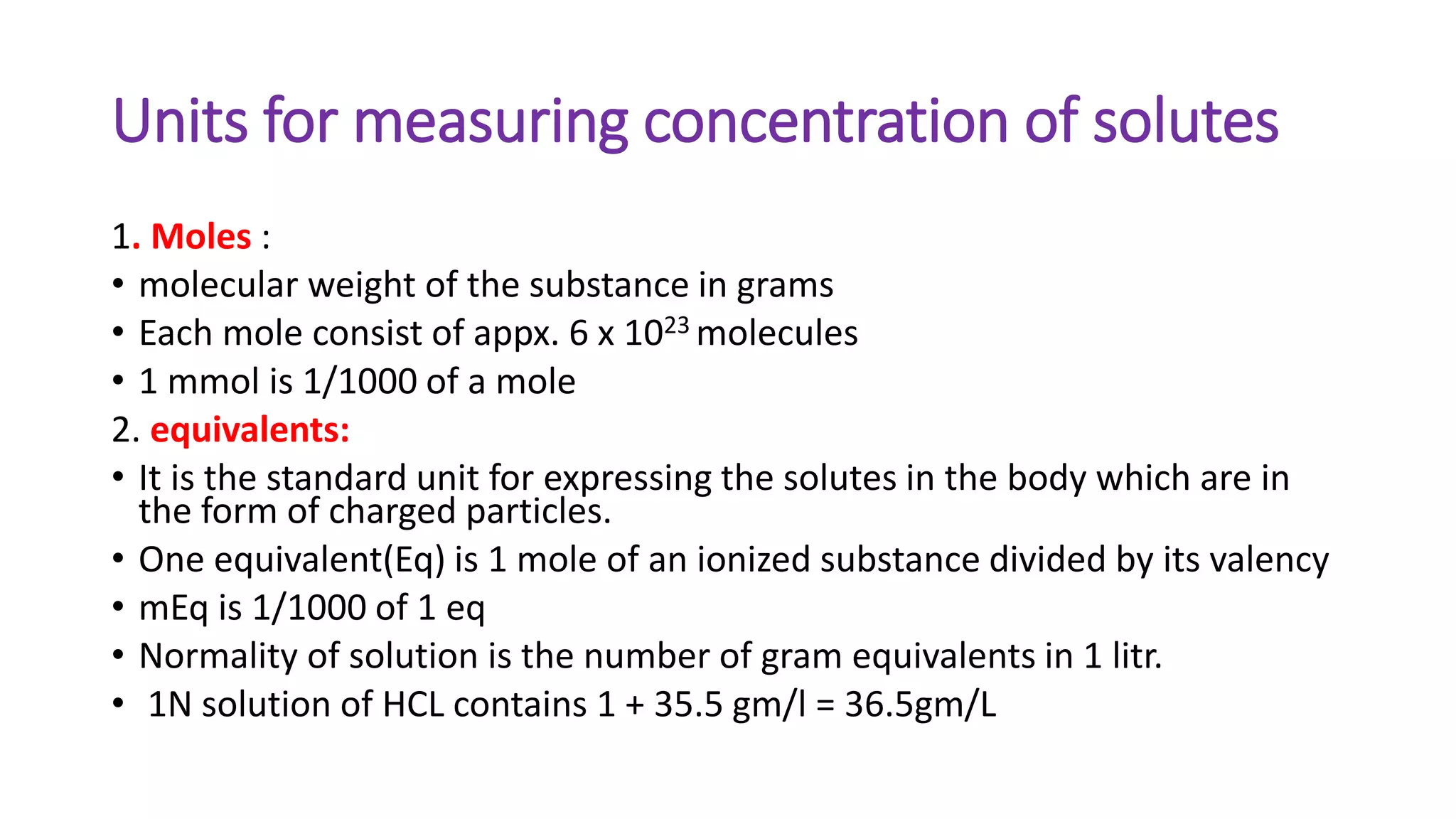
This document summarizes the composition and distribution of body fluids in the human body. It discusses that the normal adult body is composed of 60% water, 7% minerals, 18% protein, and 15% fat. Total body water is distributed between intracellular fluid (ICF, 40% of body weight) and extracellular fluid (ECF, 20% of body weight). ECF is further divided into plasma (5% of body weight), interstitial fluid (15% of body weight), and transcellular fluid (1.5% of body weight). The document also describes the ionic composition of different body fluids and units used to measure solute concentration like moles, equivalents, and osmoles. It introduces

















![Concept of pH & H+ concentration
• pH stands for power of hydrogen
• Refers to negative logarithm of the H+
i. pH= log10 1/[H+]
ii. pH= - log10[H+]
• For decrease in each pH unit (from 7 to 6, [H+] is increased 10 fold vice
versa
• pH & [H+] are inversely related
• Advantage of pH concept : when pK of buffer system is known, it is
immediately possible to determine effective pH range of the buffer
• K= ionisation or dissociation constant
• pK= negative log of K (-log K) & is equal to the pH at which half of the acid
molecules are dissociated & half undissociated](https://image.slidesharecdn.com/bodyfluidcomposition-181024112633/75/Body-fluid-amp-composition-25-2048.jpg)
![• Blood pH always refers to plasma pH(7.4)
• Range of [H+] compatible with life is 20-126 mEq/L i.e pH 7.7 to 6.9
• Optimum pH range at which human body functions properly is 7.45
• Clinically blood pH <7.35 is referred as acidosis
• Blood pH > 7.45 as alkalosis](https://image.slidesharecdn.com/bodyfluidcomposition-181024112633/75/Body-fluid-amp-composition-26-2048.jpg)
![Concept of buffer system
• A buffer is a substance that has ability to bind or release H+ in
solution
• Buffer is solution consist of a weak acid & its conjugate base, keeping
pH of solution constant
• It is a primary means by which large changes in [H+] are minimized
within a fraction of seconds](https://image.slidesharecdn.com/bodyfluidcomposition-181024112633/75/Body-fluid-amp-composition-27-2048.jpg)



