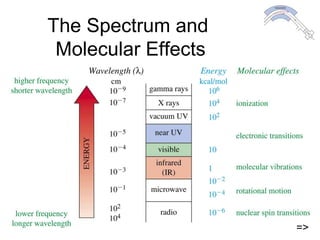
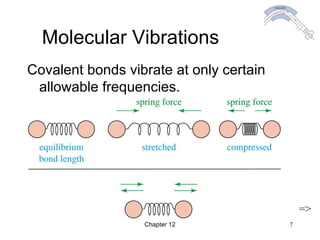
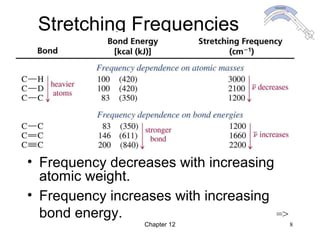
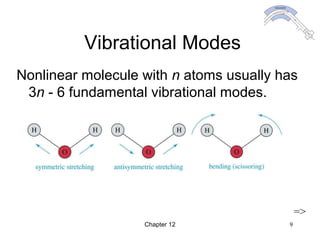
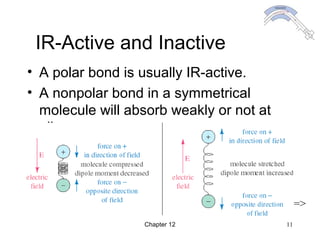
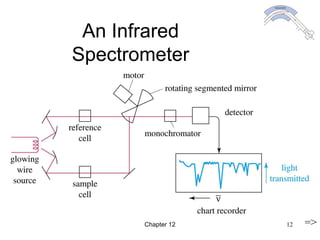
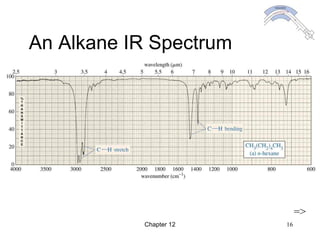
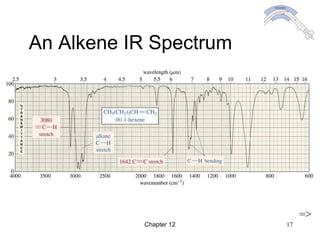
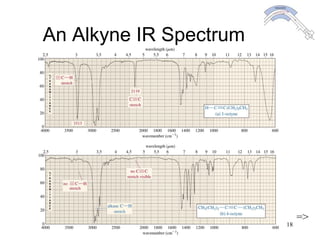
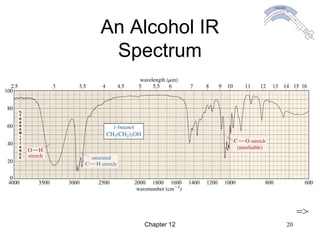
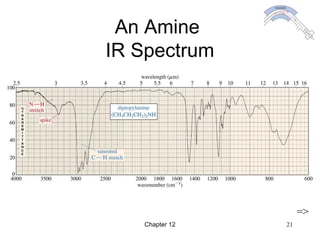
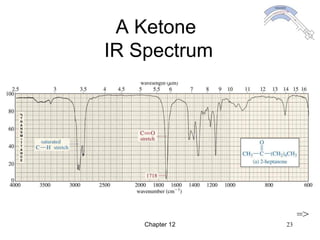
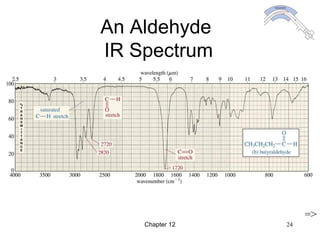
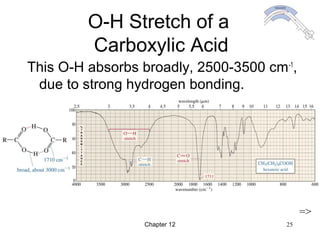
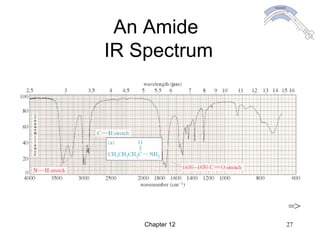
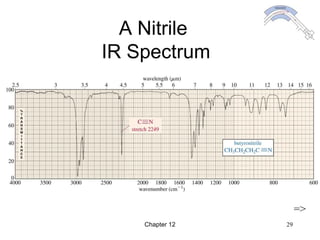
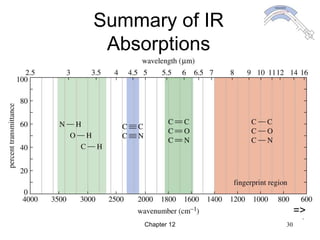
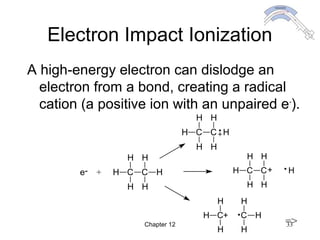
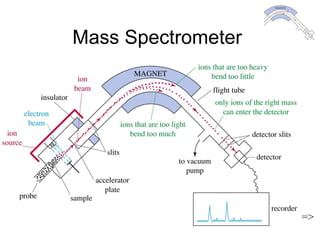
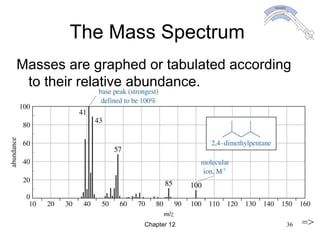
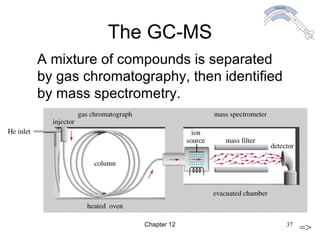
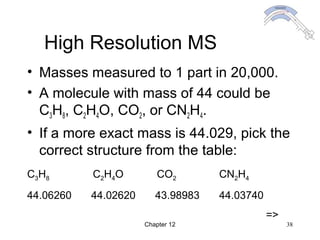
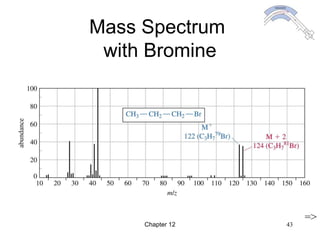
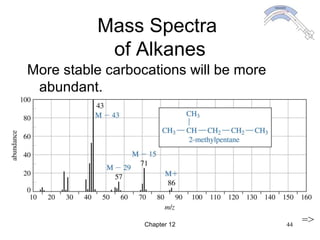
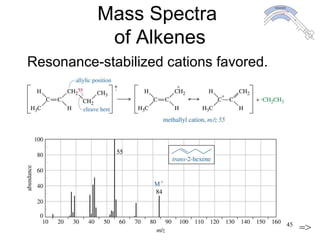
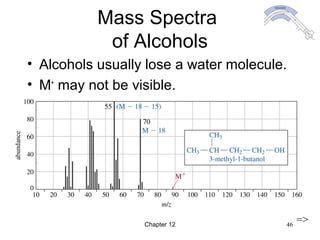
This chapter discusses infrared spectroscopy and mass spectrometry techniques for determining organic molecule structures. Infrared spectroscopy measures bond vibrations to identify functional groups present in a molecule. Mass spectrometry fragments molecules and measures the masses of the resulting fragments to deduce structural information. Together these techniques provide complementary data for elucidating molecular structures without requiring large sample amounts or destruction of the sample.